483869
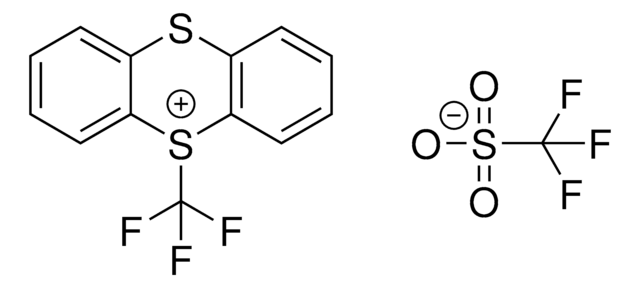
5-(Trifluoromethyl)dibenzothiophenium tetrafluoroborate
97%
Sinônimo(s):
S-(Trifluoromethyl)dibenzothiophenium tetrafluoroborate, Umemoto reagent
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C13H8BF7S
Número CAS:
Peso molecular:
340.07
Número MDL:
Código UNSPSC:
12352101
ID de substância PubChem:
NACRES:
NA.22
Ensaio:
97%
Produtos recomendados
Nível de qualidade
Ensaio
97%
pf
162-164 °C (lit.)
grupo funcional
fluoro
cadeia de caracteres SMILES
F[B-](F)(F)F.FC(F)(F)[S+]1c2ccccc2-c3ccccc13
InChI
1S/C13H8F3S.BF4/c14-13(15,16)17-11-7-3-1-5-9(11)10-6-2-4-8-12(10)17;2-1(3,4)5/h1-8H;/q+1;-1
chave InChI
VTVISWLINKWMQZ-UHFFFAOYSA-N
Aplicação
- Pd(II)-catalyzed trifluoromethylation
- Copper-catalyzed trifluoromethylation of aryl boronic acids using a Collidine as a trifluoromethylating reagent
- Pd-catalyzed electrophilic ortho-trifluoromethylation of arenes
Used in the stereoselective preparation of
- Trifluoromethyl-substituted alkenes via copper-catalyzed trifluoromethylation of terminal alkenes
- Trifluoromethyl-bearing quaternary carbon centers by Pd-catalyzed intramolecular decarboxylative allylation of α-trifluoromethyl β-keto esters
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Jun Xu et al.
Chemical communications (Cambridge, England), 47(14), 4300-4302 (2011-03-08)
A copper-catalyzed process for trifluoromethylation of aryl, heteroaryl, and vinyl boronic acids has been developed. The reaction is conducted under mild conditions and shows tolerance to moisture and a variety of functional groups.
Construction of Trifluoromethyl-Bearing Quaternary Carbon Centers by Intramolecular Decarboxylative Allylation of α-Trifluoromethyl β-Keto Esters
Shibata, N.; et al.
Advanced Synthesis & Catalysis, 353, 2037-2041 (2011)
Jun Xu et al.
Journal of the American Chemical Society, 133(39), 15300-15303 (2011-09-15)
An unprecedented type of reaction for Cu-catalyzed trifluoromethylation of terminal alkenes is reported. This reaction represents a rare instance of catalytic trifluoromethylation through C(sp(3))-H activation. It also provides a mechanistically unique example of Cu-catalyzed allylic C-H activation/functionalization. Both experimental and
Xisheng Wang et al.
Journal of the American Chemical Society, 132(11), 3648-3649 (2010-02-27)
A Pd(II)-catalyzed C-H activation/trifluoromethylation of arenes with an electrophilic trifluoromethylation reagent using diverse heterocycle directing groups is reported. The presence of trifluoroacetic acid is crucial for this catalytic reaction.
Xing-Guo Zhang et al.
Journal of the American Chemical Society, 134(29), 11948-11951 (2012-07-12)
A Pd(II)-catalyzed trifluoromethylation of ortho C-H bonds with an array of N-arylbenzamides derived from benzoic acids is reported. N-Methylformamide has been identified as a crucial promoter of C-CF(3) bond formation from the Pd center. X-ray characterization of the C-H insertion
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica