About This Item
Fórmula empírica (Notação de Hill):
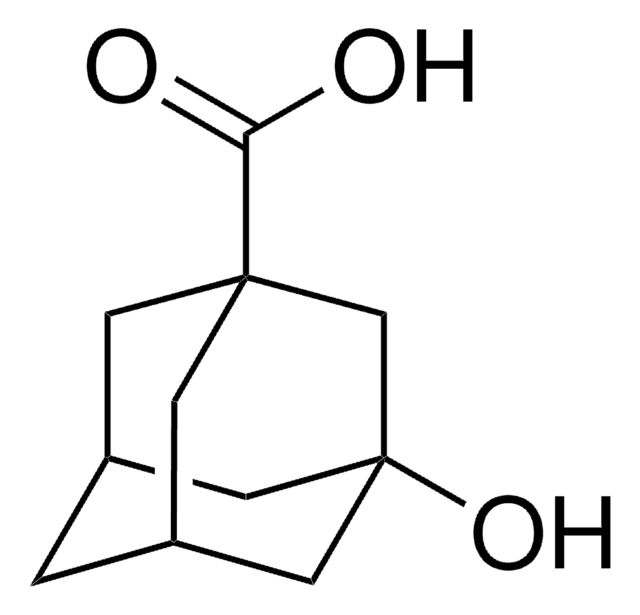
C10H14O2
Número CAS:
Peso molecular:
166.22
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
forma
solid
pf
>300 °C (lit.)
cadeia de caracteres SMILES
O[C@]12C[C@@H]3C[C@H](C1)C(=O)[C@@H](C3)C2
InChI
1S/C10H14O2/c11-9-7-1-6-2-8(9)5-10(12,3-6)4-7/h6-8,12H,1-5H2/t6-,7-,8+,10-
chave InChI
TZBDEVBNMSLVKT-XYYXLIQBSA-N
Descrição geral
5-Hydroxy-2-adamantanone is a disubstituted derivative of adamantane. The biocatalyzed synthesis of 5-hydroxy-2-adamantanone from 2-adamantanone has been investigated.
Aplicação
5-Hydroxy-2-adamantanone may be used in the following studies:
- As a model compound to investigate the application of lanthanide NMR shift reagents for the analysis of disubstituted derivative of adamantane.
- As a starting material for the synthesis of E-2-amino-5-hydroxyadamantane.
- As a starting material for the synthesis of 4-(triphenylsilyloxy)adamantan-1-ol.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
[The immunomodulator kemantan in the treatment of patients with exacerbated chronic obstructive bronchitis].
E M Rekalova
Likars'ka sprava, (4)(4), 73-76 (1992-04-01)
S S Boĭko et al.
Farmakologiia i toksikologiia, 54(1), 57-59 (1991-01-01)
The pharmacokinetics of a new Soviet-made immunostimulant kemantane, a derivative of adamantine, was studied by gas-liquid chromatography in patients with bronchial pathology. It was found that in the blood of the patients kemantane was not practically detected due to a
An expeditious preparation of E-2-amino-5-hydroxyadamantane and its Z-isomer.
Jaroskova L, et al.
Tetrahedron Letters, 47(46), 8063-8067 (2006)
Marta L Lage et al.
Tetrahedron, 69(27-28), 5609-5613 (2013-09-03)
A chemoselective method for the hydrosilylation of ketones has been developed, using the combination of triphenylsilane and a catalyst prepared from Ni(COD)
Application of shift reagents in the study of disubstituted derivatives of adamantane by NMR spectroscopy.
Vodicka L, et al.
Collection of Czechoslovak Chemical Communications, 40(1), 293-299 (1975)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica