Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
96%
Formulário
powder
pf
102-105 °C (lit.)
grupo funcional
bromo
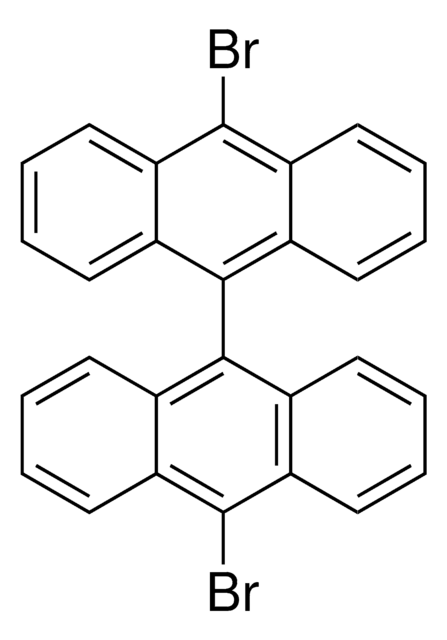
cadeia de caracteres SMILES
Brc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C16H9Br/c17-14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9H
chave InChI
HYGLETVERPVXOS-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
Aplicação
1-Bromopyrene may be used as a standard to compare its spectral properties with that of pyrene based fluorescence probe.[9] It may be used to study the effects of the addition of halogen hetero-atoms on the vapor pressures and thermodynamics of polycyclic aromatic hydrocarbons.[10]
It may be used in the synthesis of the following:
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica