346357
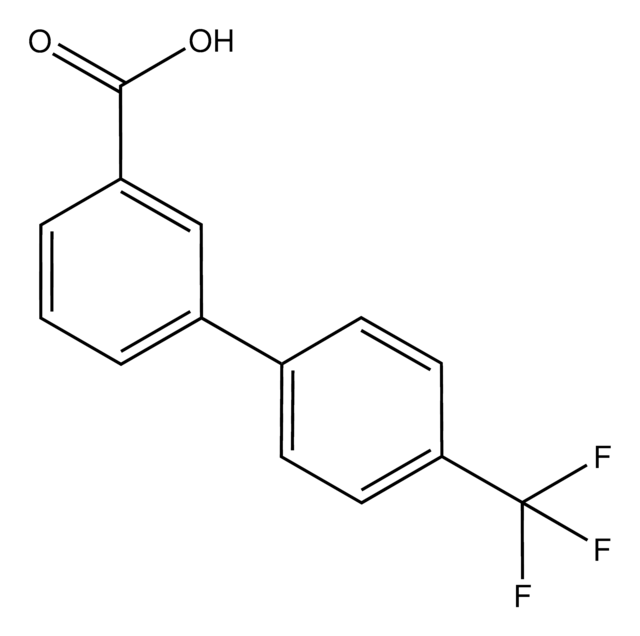
4′-(Trifluoromethyl)-2-biphenylcarboxylic acid
97%
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CF3C6H4C6H4CO2H
Número CAS:
Peso molecular:
266.22
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
forma
solid
pf
169-171 °C (lit.)
grupo funcional
carboxylic acid
fluoro
cadeia de caracteres SMILES
OC(=O)c1ccccc1-c2ccc(cc2)C(F)(F)F
InChI
1S/C14H9F3O2/c15-14(16,17)10-7-5-9(6-8-10)11-3-1-2-4-12(11)13(18)19/h1-8H,(H,18,19)
chave InChI
IQOMYCGTGFGDFN-UHFFFAOYSA-N
Descrição geral
4′-(Trifluoromethyl)-2-biphenylcarboxylic acid (xenalipin) has been tested as an effective hypolipidemic agent in animal species. It has been shown to cause significant reduction in serum cholesterol and triglyceride levels in animal models and would be beneficial in therapy for hyperlipidemia. Synthesis of 4′-(trifluoromethyl)-2-biphenylcarboxylic acid (xenalipin) has been reported. Xenalipin has been synthesized in the [14C]-labelled form, with specific activity 21.0mCi/mmol, which is suitable for the metabolism and distribution studies in animals.
Aplicação
4′-(Trifluoromethyl)-2-biphenylcarboxylic acid may be used in chemical synthesis.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
M C Lewis et al.
Atherosclerosis, 64(1), 27-35 (1987-03-01)
Xenalipin (4'-trifluoromethyl-2-biphenylcarboxylic acid) is a chemically novel compound which has been found to be an effective hypolipidemic agent in two animal species. Significant reductions in serum cholesterol and triglycerides were observed in cholesterol-cholic acid-fed rats following oral doses of 10-30
Synthesis of carbon-14 labelled xenalipin-a potential hypolipidemic agent.
Hill JA and Eaddy JF.
Journal of Labelled Compounds & Radiopharmaceuticals, 31(12), 1011-1017 (1992)
The Nickel (I) Catalyzed Coupling of a Diarylzinc with an Aryl Chloride in the Synthesis of Xenalipin.
Eaddy JF.
Organic preparations and procedures international, 27(3), 367-372 (1995)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica