About This Item
Produtos recomendados
Ensaio
98%
Formulário
powder
p.e.
180-184 °C/15 mmHg (lit.)
grupo funcional
hydroxyl
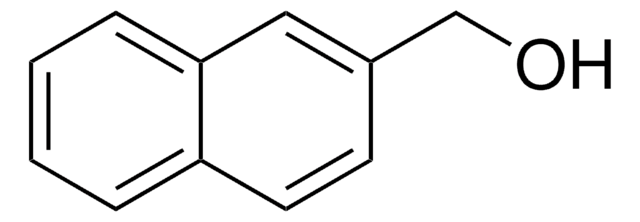
cadeia de caracteres SMILES
OCCc1ccc2ccccc2c1
InChI
1S/C12H12O/c13-8-7-10-5-6-11-3-1-2-4-12(11)9-10/h1-6,9,13H,7-8H2
chave InChI
VCZANYLMPFRUHG-UHFFFAOYSA-N
Informações sobre genes
human ... BAD(572)
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica