803448
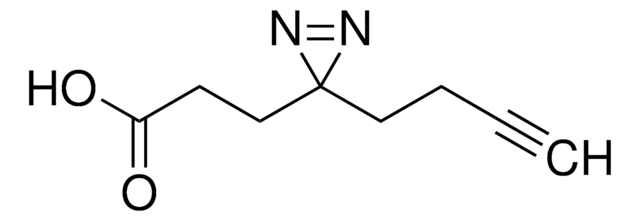
SDAD (NHS-SS-Diazirine) (succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithiopropionate)
About This Item
Empfohlene Produkte
Assay
≥90%
Form
powder
Mol-Gew.
388.46
Eignung der Reaktion
reagent type: cross-linking reagent
Lagerbedingungen
desiccated
Löslichkeit
DMSO or DMF: soluble
Versandbedingung
ambient
Lagertemp.
2-8°C
SMILES String
CC1(N=N1)CCC(NCCSSCCC(ON2C(CCC2=O)=O)=O)=O
InChI
1S/C14H20N4O5S2/c1-14(16-17-14)6-4-10(19)15-7-9-25-24-8-5-13(22)23-18-11(20)2-3-12(18)21/h2-9H2,1H3,(H,15,19)
InChIKey
NLPWBELUEANJAT-UHFFFAOYSA-N
Allgemeine Beschreibung
Leistungsmerkmale und Vorteile
- Membrane-permeable—suitable for in vivo intracellular protein crosslinking
- Heterobifunctional—NHS ester group reacts with primary amines at pH 7 to 9 to form covalent amide bonds; diazirine (azipentanoate) group reacts efficiently with any amino acid side chain or peptide backbone upon activation with long-wave UV light (330-370 nm)
- Controllable—two-step chemical crosslinking is activated using common laboratory UV lamps
- Easy to use—these crosslinkers are photo-stable under typical laboratory lighting conditions so there is no need to perform experiments in the dark
- Better than aryl azides—the diazirine photoreactive group has better photostability in normal light than phenyl azide groups of traditional photoreactive crosslinkers, yet the diazirine group is more efficiently activated by long-wave UV light
Vorsicht
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.