Wichtige Dokumente
706531
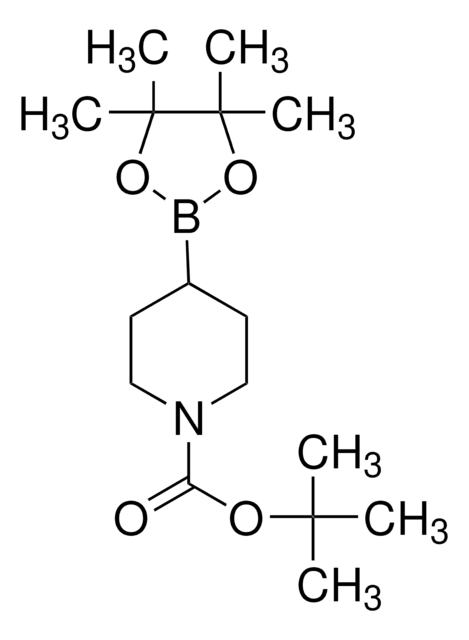
N-Boc-1,2,3,6-Tetrahydropyridin-4-Boronsäurepinakolester
95%
Synonym(e):
(N-tert.-Butoxycarbonyl)-1,2,3,6-tetrahydropyridin-4-boronsäure-pinakolester
Größe auswählen
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
95%
Form
powder
mp (Schmelzpunkt)
100-114 °C
SMILES String
CC(C)(C)OC(=O)N1CCC(=CC1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C16H28BNO4/c1-14(2,3)20-13(19)18-10-8-12(9-11-18)17-21-15(4,5)16(6,7)22-17/h8H,9-11H2,1-7H3
InChIKey
VVDCRJGWILREQH-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- Suzuki-Miyaura cross-coupling using palladium phosphine catalyst[1]
- Palladium-catalyzed ligand-controlled regioselective Suzuki coupling[2]
- Palladium-catalyzed Suzuki-Miyaura coupling[3]
- Suzuki coupling followed by iodolactonization reaction[4]
- Wrenchnolol derivative optimized for gene activation in cells[5]
Reagent used in Preparation of several enzymatic inhibitors and receptor ligands
- Orally active anaplastic lymphoma kinase inhibitors[6]
- Oxazolecarboxamides as diacylglycerol acyltransferase-1 inhibitors for treatment of obesity and diabetes[7]
- 4-arylpiperidinyl amides and N-arylpiperidin-3-yl-cyclopropanecarboxamides as novel melatonin receptor ligands[8]
- Quinazoline analogs as glucocerebrosidase inhibitors with chaperone activity for treatment of Gaucher disease, a lysosomal storage disorder[9]
- Arylpiperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists[10]
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Active Filters
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)



![[1,1′-Bis(diphenylphosphino)ferrocen]dichlorpalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

