632961
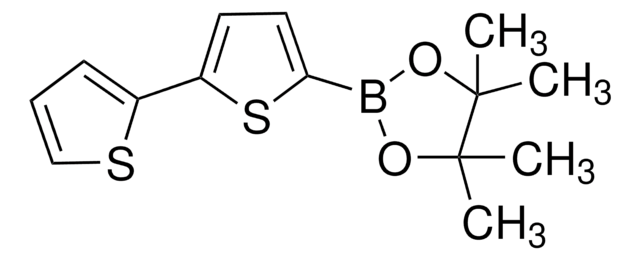
5′-Hexyl-2,2′-bithiophen-5-Boronsäurepinakolester
97%
Synonym(e):
5′-N-Hexyl-2,2′-bithiophen-5-boronsäure-pinakolester, 5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-5′-N-hexyl-2,2′-bithiophen, 5-Hexyl-5′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,2′-bithiophen
About This Item
Empfohlene Produkte
Assay
97%
Form
solid
mp (Schmelzpunkt)
36-40 °C (lit.)
SMILES String
CCCCCCc1ccc(s1)-c2ccc(s2)B3OC(C)(C)C(C)(C)O3
InChI
1S/C20H29BO2S2/c1-6-7-8-9-10-15-11-12-16(24-15)17-13-14-18(25-17)21-22-19(2,3)20(4,5)23-21/h11-14H,6-10H2,1-5H3
InChIKey
XTTRNSNHDCYSEL-UHFFFAOYSA-N
Anwendung
- Suzuki-Miyaura cross-coupling reactions and shape-shifting in contorted dibenzotetrathienocoronenes
- Oligothiophene self-assembly induction into fibers with tunable shape and function
- Stille coupling and p-conjugated packing structure and hole mobility of bithiophene-bithiazole copolymers with alkyl-thiophene side chains
Reagent used in Preparation of
- Solution-processed ambipolar field-effect transistor
- Light harvesting small molecules for use in solution-processed small molecule bulk heterojunction solar cell devices
- Light-emitting diode (OLED) materials
- Unsymmetric substituted benzothiadiazole-containing vinyl monomers for RAFT polymerization
- Pd-catalyzed condensations and synthesis of isoindigo-based oligothiophenes for molecuar bulk heterojunction solar cells
- Thiophene-benzothiadiazole based donor-acceptor-donor materials
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.