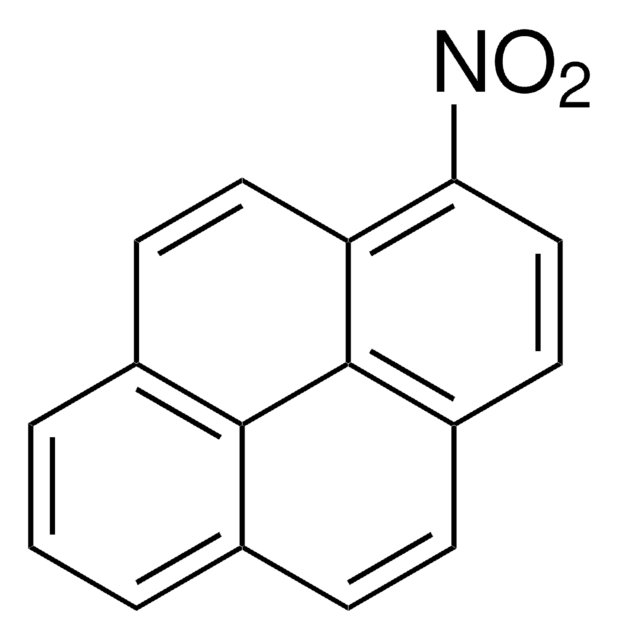
149101
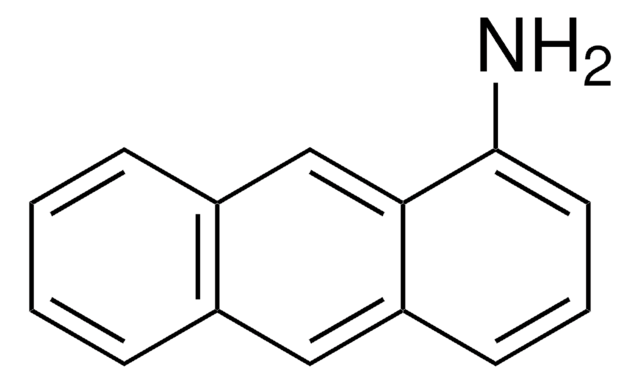
9-Aminophenanthrene
96%
Synonym(s):
9-Phenanthrenamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H11N
CAS Number:
Molecular Weight:
193.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
137-139 °C (lit.)
SMILES string
Nc1cc2ccccc2c3ccccc13
InChI
1S/C14H11N/c15-14-9-10-5-1-2-6-11(10)12-7-3-4-8-13(12)14/h1-9H,15H2
InChI key
KIHQWOBUUIPWAN-UHFFFAOYSA-N
Application
9-Aminophenanthrene was used as a model ligand to study the binding of 9-aminophenanthrene to individual subsites within the active site of CYP(eryF).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kishore K Khan et al.
Chemical research in toxicology, 15(6), 806-814 (2002-06-18)
The main objective of the present study was to find a fluorescent substrate probe for cytochrome P450eryF (P450eryF). P450eryF is a bacterial P450 that catalyzes the hydroxylation of 6-deoxyerythronolide B at the 6S position, a necessary step in the biosynthesis
Moon-Young Yoon et al.
Drug metabolism reviews, 36(2), 219-230 (2004-07-09)
Cytochrome P450-dependent drug metabolism in vitro frequently deviates from simple Michaelis-Menten kinetic models, and demonstrates both positive and negative homotropic and heterotropic effects. These complex "allsoteric" kinetics confound our ability to predict drug clearance, and they may provide a basis
H Y Aboul-Enein et al.
The Journal of pharmacy and pharmacology, 50(3), 291-296 (1998-05-26)
Racemic ketoprofen is a non-steroidal anti-inflammatory drug used to treat musculoskeletal and colic conditions in horses. The enantioselective chiral inversion of ketoprofen administered to horses has been studied by use of cellulose tris(4-methylbenzoate), also known as Chiralcel OJ-R, as chiral
Arthur G Roberts et al.
Biochemistry, 45(6), 1673-1684 (2006-02-08)
Cytochrome P450's (P450's) catalyze the oxidative metabolism of most drugs and toxins. Although extensive studies have proven that some P450's demonstrate both homotropic and heterotropic cooperativity toward a number of substrates, the mechanistic and molecular details of P450 allostery are
M Ikeda et al.
Journal of chromatography, 305(2), 261-270 (1984-02-10)
The application of 9-aminophenanthrene (9-AP), a fluorescence-labeling reagent for free fatty acids (FFA), was examined. 9-AP dissolved in benzene was added to a benzene solution of FFA chlorides derived from FFA and oxalyl chloride. The mixture was allowed to react
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service