903000
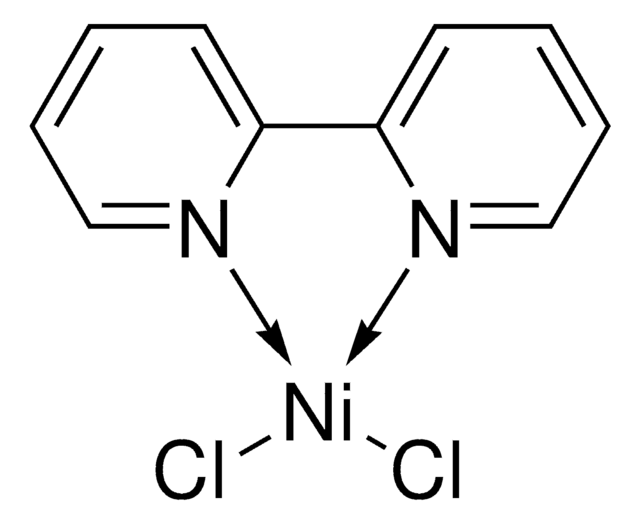
[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride
Synonym(s):
(4,4′-dtbbpy)NiCl2
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C18H24Cl2N2Ni
CAS Number:
Molecular Weight:
398.00
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
form
powder or crystals
reaction suitability
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
mp
>300 °C
SMILES string
CC(C1=CC(C2=CC(C(C)(C)C)=CC=N2)=NC=C1)(C)C.Cl[Ni]Cl
Application
[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride can be used as a catalyst in:
- Decarboxylative arylation of oxo acids.
- Acylation of ethers.
- Cross-coupling of aryl bromides with alcohols.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Photocatalytic α-Acylation of Ethers
Sun Z, et al.
Organic Letters, 19, 3727-3730 (2017)
Aryl Ketones as Single-Electron-Transfer Photoredox Catalysts in Nickel-Catalyzed the Homocoupling of Aryl Halides
Masuda Y, et al.
European Journal of Organic Chemistry, 5822-5825 (2016)
Martins S Oderinde et al.
The Journal of organic chemistry, 80(15), 7642-7651 (2015-07-04)
In order to achieve reproducibility during iridium-photoredox and nickel dual-catalyzed sp(3)-sp(2) carbon-carbon bond-forming reactions, we investigated the role that molecular oxygen (O2), solvent and light-source (CF lamp or blue LED) play in a variety of Ir-photoredox mediated transformations. The presence
Merging photoredox and nickel catalysis: The direct synthesis of ketones by the decarboxylative arylation of α-oxo acids
Chu L, et al.
Angewandte Chemie (International ed. in English), 127, 8040-8044 (2015)
Ir III/Ni II-Metallaphotoredox catalysis: the oxidation state modulation mechanism versus the radical mechanism
Zhu B, et al.
Chemical Communications (Cambridge, England), 54, 5968-5971 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)



![[Ni(dtbbpy)(H2O)4]Cl2](/deepweb/assets/sigmaaldrich/product/structures/777/629/15c13300-e874-4abd-8bd4-8b2bb4864570/640/15c13300-e874-4abd-8bd4-8b2bb4864570.png)

![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)

![Bis[(2-dimethylamino)phenyl]amine nickel(II) chloride ≥97% (AT)](/deepweb/assets/sigmaaldrich/product/structures/143/670/3d0cc911-c810-4324-914e-85c5c11b7dac/640/3d0cc911-c810-4324-914e-85c5c11b7dac.png)
![[(TMEDA)Ni(o-tolyl)Cl] 95%](/deepweb/assets/sigmaaldrich/product/structures/236/439/768c916e-994f-47e3-a980-3ca0471317d7/640/768c916e-994f-47e3-a980-3ca0471317d7.png)



![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)
![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)