推薦產品
等級
pharmaceutical primary standard
API 家族
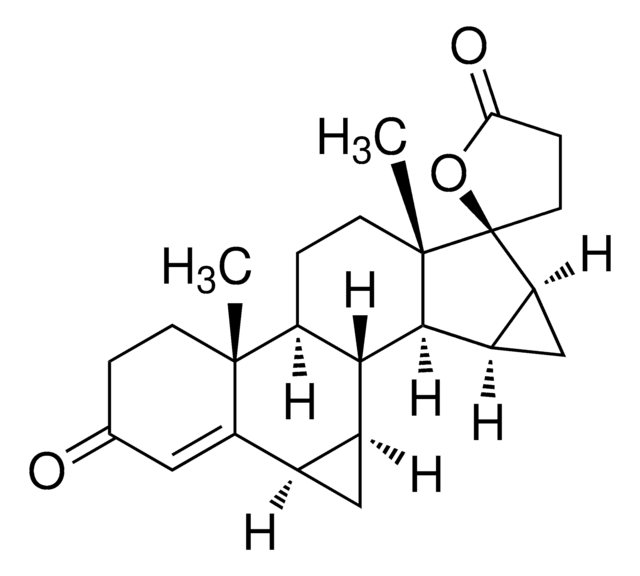
medroxyprogesterone
製造商/商標名
USP
mp
206-207 °C (lit.)
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
[H][C@@]12C[C@H](C)C3=CC(=O)CC[C@]3(C)[C@@]1([H])CC[C@@]4(C)[C@@]2([H])CC[C@]4(OC(C)=O)C(C)=O
InChI
1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3/t14-,18+,19-,20-,22+,23-,24-/m0/s1
InChI 密鑰
PSGAAPLEWMOORI-PEINSRQWSA-N
基因資訊
human ... PGR(5241)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Medroxyprogesterone acetate USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Medroxyprogesterone Acetate Injectable Suspension
- Medroxyprogesterone Acetate Tablets
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 4 - Carc. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
J Mitsushita et al.
Gynecologic oncology, 79(1), 129-132 (2000-09-28)
Successful pregnancies after conservative progestin treatment to young women with endometrial carcinoma have recently been reported. However, it is not known for certain whether the lesion is completely eradicated in such patients. We present a case of residual endometrial carcinoma
Edith R Guilbert et al.
Contraception, 79(3), 167-177 (2009-02-03)
In the fall of 2007, the controversy about the contraceptive use of depot-medroxyprogesterone acetate (DMPA) and its potential impact on skeletal health reached the media in the province of Quebec, Canada, thereby becoming a matter of concern for the lay
Carolyn Westhoff
Contraception, 68(2), 75-87 (2003-09-05)
Depot-medroxyprogesterone acetate (Depo-Provera(R)) is a highly effective, nondaily hormonal contraceptive option that has been available in the United States for a decade, and worldwide for 40 years. Benefits and risks of hormonal therapy are often under scrutiny; however, long-term clinical
Andrea N Simpson et al.
Gynecologic oncology, 133(2), 229-233 (2014-02-25)
Oral progestin is an alternative to hysterectomy for women with complex atypical hyperplasia (CAH) or grade one endometrial cancer (G1EC) who wish fertility preservation. We evaluated treatment efficacy and fertility outcomes in this population. Women <45 y treated with oral
Summer Day et al.
Journal of acquired immune deficiency syndromes (1999), 66(4), 452-456 (2014-05-07)
Depot medroxyprogesterone acetate (DMPA) use among HIV-1-infected women may increase transmission by increasing plasma and genital HIV-1 RNA shedding. We investigated associations between DMPA use and HIV-1 RNA in plasma and cervical secretions. One hundred two women initiated antiretroviral therapy
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務