推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
powder
顏色
white
溶解度
DMSO: 9 mg/mL
起源
Novartis
儲存溫度
−20°C
SMILES 字串
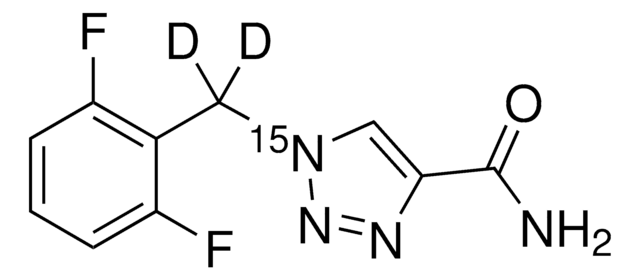
NC(=O)c1cn(Cc2c(F)cccc2F)nn1
InChI
1S/C10H8F2N4O/c11-7-2-1-3-8(12)6(7)4-16-5-9(10(13)17)14-15-16/h1-3,5H,4H2,(H2,13,17)
InChI 密鑰
POGQSBRIGCQNEG-UHFFFAOYSA-N
基因資訊
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
尋找類似的產品? 前往 產品比較指南
應用
Rufinamide has been used to test its analgesic effect on neuropathic pain in spared nerve injury (SNI) model.[1]
生化/生理作用
Broad-spectrum anticonvulsant.
Rufinamide may elicit inhibition of the sodium channels and block action potential generation. This property makes it an antiepileptic drug for treating epilepsy disorders like Lennox-Gastaut syndrome.[1]
特點和優勢
This compound was developed by Novartis. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
診斷意義
Effective therapy for partial seizures in adults and childhood seizures in Lennox-Gastaut syndrome.
訊號詞
Warning
危險聲明
危險分類
Carc. 2 - Repr. 2 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
客戶也查看了
Shin Hye Kim et al.
Seizure, 21(4), 288-291 (2012-03-17)
To evaluate the efficacy of rufinamide as an add-on treatment in children and adolescents with Lennox-Gastaut syndrome (LGS). The study was an open-label, observational clinical trial of rufinamide as an add-on treatment in intractable LGS patients. This intent-to-treat trial included
Iolanda Mazzucchelli et al.
Analytical and bioanalytical chemistry, 401(3), 1013-1021 (2011-06-07)
The development of a simple and rapid high-performance liquid chromatography (HPLC) method for the determination of the new antiepileptic drug rufinamide (RFN) in human plasma and saliva is reported. Samples (250 μl) are alkalinized with ammonium hydroxide (pH 9.25) and
Emma D Deeks et al.
CNS drugs, 20(9), 751-760 (2006-09-07)
Rufinamide, a triazole derivative, reduces the recovery capacity of neuronal sodium channels after inactivation, limiting neuronal sodium-dependent action potential firing and mediating anticonvulsant effects. In children, adolescents and adults, adjunctive oral rufinamide was more effective than placebo in treating seizures
K K Jain
Expert opinion on investigational drugs, 9(4), 829-840 (2000-11-04)
This article evaluates rufinamide, a new anti-epileptic drug (AED) in Phase III development. This review is done against the background of therapeutic challenges of epilepsy, old established AEDs, newly introduced AEDs and AEDs in clinical development. Pharmacological properties of 12
M Häusler et al.
Neuropediatrics, 42(1), 28-29 (2011-05-11)
Epilepsy with myoclonic absences (EMA) is a rare epileptic syndrome with frequently poor response to antiepileptic treatment. Rufinamide (RUF) is a relatively new EMEA- and FDA-approved anticonvulsant licensed as an orphan drug for the adjunctive treatment of patients with Lennox-Gastaut
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務