全部照片(4)
About This Item
經驗公式(希爾表示法):
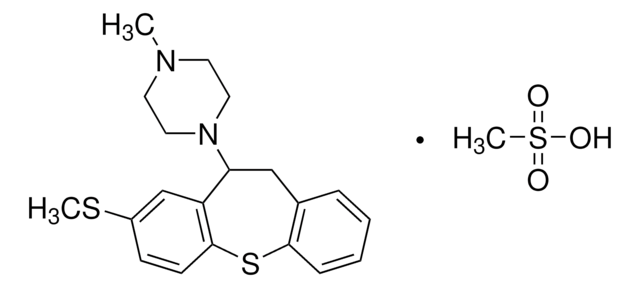
C18H20N2 · HCl
CAS號碼:
分子量::
300.83
EC號碼:
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
暫時無法取得訂價和供貨情況
推薦產品
溶解度
H2O: 3.4 mg/mL
ethanol: 5.6 mg/mL
起源
Organon
儲存溫度
2-8°C
SMILES 字串
Cl.CN1CCN2C(C1)c3ccccc3Cc4ccccc24
InChI
1S/C18H20N2.ClH/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19;/h2-9,18H,10-13H2,1H3;1H
InChI 密鑰
YNPFMWCWRVTGKJ-UHFFFAOYSA-N
基因資訊
human ... ADRA2A(150) , ADRA2B(151) , ADRA2C(152) , HRH1(3269) , HTR2A(3356) , HTR2B(3357) , HTR2C(3358)
尋找類似的產品? 前往 產品比較指南
應用
Mianserin hydrochloride has been used:
- as a reversible antagonist for serotonergic -protein coupled receptor (GPCR) - G-protein protein-coupled receptor (S7.1R)[1]
- as an antidepressant in hippocampal astrocytes to test its effect on brain-derived neurotrophic factor (BDNF) mRNA expression[2]
- as a 5-hydroxytryptamine (5-HT) receptor antagonist to study its effect on serotonin modulation[3]
生化/生理作用
Antidepressant; antagonist/inverse agonist at 5-HT2 serotonin receptors; also blocks the H1 histamine receptor and the α2 adrenoceptor.
特點和優勢
This compound was developed by Organon. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
準備報告
Solutions may be stored for several days at 4°C.
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
客戶也查看了
I M Leitch et al.
The Journal of pharmacy and pharmacology, 44(4), 315-320 (1992-04-01)
Some in-vitro pharmacological effects of a novel analogue of mianserin, 2-carboxamidino-1,2,3,4,10,14b-hexahydrodibenzo (c,f) pyrazino (1,2-alpha) azepine hydrochloride (FCC5) have been studied. FCC5 was a non-competitive antagonist of both histamine-induced contractions of the guinea-pig ileum and 5-HT-induced contractions of rat fundal strips
T de Boer
International clinical psychopharmacology, 10 Suppl 4, 19-23 (1995-12-01)
Mirtazapine is a new antidepressant with a unique mode of action: it preferentially blocks the noradrenergic alpha2-auto- and heteroreceptors held responsible for controlling noradrenaline and serotonin release. In addition, mirtazapine has a low affinity for serotonin (5-HT)1A receptors but potently
Modulatory effects of the serotonergic and histaminergic systems on reaction to light in the crustacean Gammarus pulex
Perrot-Minnot MJ, et al.
Neuropharmacology, 75, 31-37 (2013)
Anti-depressant fluoxetine reveals its therapeutic effect via astrocytes
Kinoshita M, et al.
EBioMedicine, 32(3), 72-83 (2018)
D L Willins et al.
Neuroscience, 91(2), 599-606 (1999-06-12)
In this study, we demonstrate that clozapine and other atypical antipsychotic drugs induce a paradoxical internalization of 5-hydroxytryptamine-2A receptors in vitro and a redistribution of 5-hydroxytryptamine-2A receptors in vivo. We discovered that clozapine, olanzapine, risperidone and the putative atypical antipsychotic
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務