全部照片(1)
About This Item
經驗公式(希爾表示法):
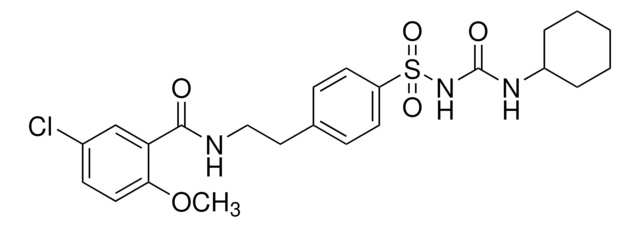
C24H34N4O5S
CAS號碼:
分子量::
490.62
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推薦產品
化驗
≥98% (HPLC)
形狀
solid
顏色
white
mp
212.2-214.5 °C
溶解度
DMSO: >10 mg/mL
起源
Sanofi Aventis
儲存溫度
room temp
SMILES 字串
CCC1=C(C)CN(C(=O)NCCc2ccc(cc2)S(=O)(=O)NC(=O)N[C@H]3CC[C@H](C)CC3)C1=O
InChI
1S/C24H34N4O5S/c1-4-21-17(3)15-28(22(21)29)24(31)25-14-13-18-7-11-20(12-8-18)34(32,33)27-23(30)26-19-9-5-16(2)6-10-19/h7-8,11-12,16,19H,4-6,9-10,13-15H2,1-3H3,(H,25,31)(H2,26,27,30)/t16-,19-
InChI 密鑰
WIGIZIANZCJQQY-RUCARUNLSA-N
基因資訊
human ... ABCC8(6833) , KCNJ1(3758) , KCNJ11(3767)
尋找類似的產品? 前往 產品比較指南
一般說明
格列美脲属于生物制药II类二代磺酰脲类药物。
應用
格列美脲已被用于:
- 作为一种降糖药物用于测试其在人脐静脉细胞(HUVEC)中的抗糖尿病功能
- 在乳腺癌MDA-MB-231细胞中作为磺酰脲类ATP敏感型钾通道(KATP)抑制剂
- 在新生儿胰岛样细胞簇(NICC)的葡萄糖刺激胰岛素分泌(GSIS)测定中检测其对胰岛素分泌的影响
格列美脲目前用于治疗 2 型糖尿病。
生化/生理作用
格列美脲可通过刺激胰腺β细胞分泌胰岛素激素来降低血糖水平。它可与与β细胞相关的一种65-kD蛋白发生相互作用。
格列美脲是一种有效的心肌梗死 KATP 通道阻断剂,被吡那地尔激活,IC50 值为 6.8 nM。
特點和優勢
该化合物由Sanofi Aventis开发。要浏览其他药物开发化合物和批准的药物/候选药物列表,单击此处。
訊號詞
Warning
危險聲明
危險分類
Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Li-Ping Wang et al.
Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 38(6), 2337-2347 (2016-05-21)
By inducing severe endothelial impairment, hypertension and diabetes are two leading causes of morbidity and mortality. Hypertensive patients with concomitant diabetes must take both antihypertensive and hypoglycaemic medications, for which there is a lack of experimental and clinical guidelines. This
A REVIEW ARTICLE ON GLIMEPERIDE: AN ORAL HYPOGLYCAEMIC DRUG
Tiwari A, et al.
International Journal of Advanced Research , 4, 920-927 (2016)
William T Cefalu et al.
Lancet (London, England), 382(9896), 941-950 (2013-07-16)
Sodium-glucose cotransporter 2 (SGLT2) inhibitors improve glycaemia in patients with type 2 diabetes by enhancing urinary glucose excretion. We compared the efficacy and safety of canagliflozin, an SGLT2 inhibitor, with glimepiride in patients with type 2 diabetes inadequately controlled with
W Rathmann et al.
Diabetes, obesity & metabolism, 15(1), 55-61 (2012-08-07)
To investigate therapy persistence, frequency of hypoglycaemia and macrovascular outcomes among type 2 diabetes patients with dipeptidyl peptidase-4 (DPP-4) inhibitors (DPP-4) and sulphonylureas (SU). Data from 19,184 DPP-4 (mean age: 64 years; 56% males) and 31,110 SU users (69 years;
Mitsuyoshi Takahara et al.
Endocrine journal, 59(12), 1131-1136 (2012-08-02)
We retrospectively investigated the effect of adding dipeptidyl peptidase-4 (DPP-4) inhibitor and tapering sulfonylurea on blood glucose fluctuation in Asian patients with type 2 diabetes mellitus under basal-supported oral therapy (BOT). We recruited twenty-two consecutive Japanese patients with type 2
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務