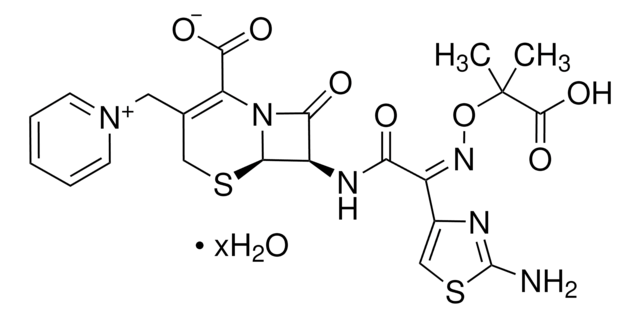
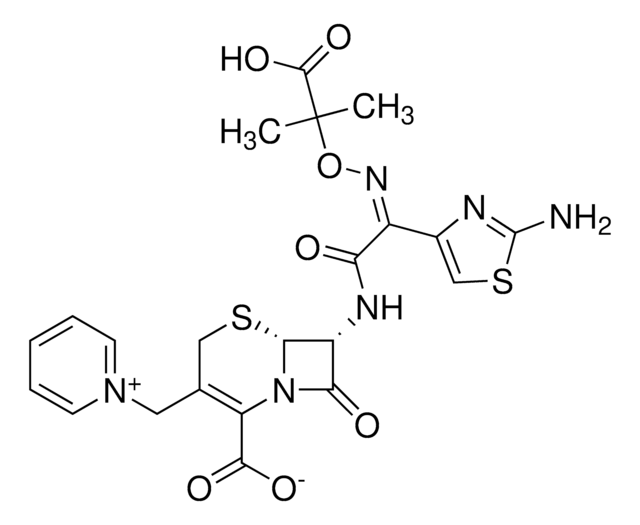
This product is not tested for solubility. However, information available in the literature states that Ceftazidime hydrate is soluble in 0.1 M NaOH at 50 mg/mL. This information has not been validated.
推薦產品
描述
does not contain sodium carbonate
品質等級
化驗
95.0-102.0% anhydrous basis
形狀
powder or crystals
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
作用方式
cell wall synthesis | interferes
儲存溫度
2-8°C
SMILES 字串
[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].CC(C)(O\N=C(/C(=O)N[C@H]1[C@H]2SCC(C[n+]3ccccc3)=C(N2C1=O)C([O-])=O)c4csc(N)n4)C(O)=O
InChI
1S/C22H22N6O7S2.5H2O/c1-22(2,20(33)34)35-26-13(12-10-37-21(23)24-12)16(29)25-14-17(30)28-15(19(31)32)11(9-36-18(14)28)8-27-6-4-3-5-7-27;;;;;/h3-7,10,14,18H,8-9H2,1-2H3,(H4-,23,24,25,29,31,32,33,34);5*1H2/b26-13-;;;;;/t14-,18-;;;;;/m1...../s1
InChI 密鑰
NMVPEQXCMGEDNH-TZVUEUGBSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
生化/生理作用
其他說明
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
從最近期的版本中選擇一個:
分析證明 (COA)
客戶也查看了
-
What is the solubility of this product?
1 answer-
Helpful?
-
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務