全部照片(1)
About This Item
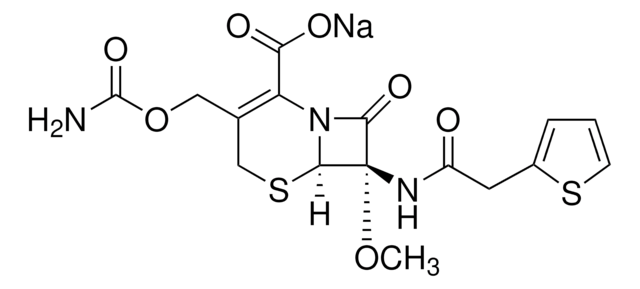
經驗公式(希爾表示法):
C16H16N5NaO7S2
CAS號碼:
分子量::
477.45
Beilstein:
5711411
EC號碼:
MDL號碼:
分類程式碼代碼:
51284136
PubChem物質ID:
NACRES:
NA.85
推薦產品
形狀
powder
品質等級
效力
916-964 μg per mg
溶解度
H2O: 50 mg/mL
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
作用方式
cell wall synthesis | interferes
儲存溫度
2-8°C
SMILES 字串
[Na+].[H][C@]12SCC(COC(C)=O)=C(N1C(=O)[C@H]2NC(=O)C(=N/OC)\c3csc(N)n3)C([O-])=O
InChI
1S/C16H17N5O7S2.Na/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8;/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26);/q;+1/p-1/b20-9-;/t10-,14-;/m1./s1
InChI 密鑰
AZZMGZXNTDTSME-JUZDKLSSSA-M
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
Chemical structure: ß-lactam
應用
Cefotaxim has been used to find new residues involved in cefotaxime hydrolysis in CTX-M β-lactamases, to study antibiotic-susceptible and -resistant Streptococcus pneumoniae infections, and to study pneumococcal pneumonia and the pharmokinetics of various treatments. It may be used to study the effects of binding and inhibition of penicillin binding protein 2 (PBP2) on bacterial mucopeptide synthesis.
生化/生理作用
Cefotaxim inhibits bacterial cell wall synthesis by binding to penicillin-binding proteins (PBPs) which inhibits the final transpeptidation step of peptidoglycan synthesis in bacterial cell walls. As a result, bacteria lyse due to cell wall autolytic enzymes.
其他說明
Broad spectrum third generation cephalosporin antibiotic.
Keep container tightly closed in a dry and well-ventilated place.
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Violeta Rodríguez-Cerrato et al.
The Journal of antimicrobial chemotherapy, 60(5), 1159-1162 (2007-09-11)
In an innovative therapeutic exploitation against antibiotic-resistant Streptococcus pneumoniae, here we have evaluated the in vitro activity of a purified bacterially-encoded cell wall lytic enzyme, LytA (the major pneumococcal autolysin), and compared it with those of Cpl-1 and Pal (pneumococcal
Francisco José Pérez-Llarena et al.
Antimicrobial agents and chemotherapy, 55(9), 4361-4368 (2011-07-07)
The CTX-M β-lactamases are an increasingly prevalent group of extended-spectrum β-lactamases (ESBL). Point mutations in CTX-M β-lactamases are considered critical for enhanced hydrolysis of cefotaxime. In order to clarify the structural determinants of the activity against cefotaxime in CTX-M β-lactamases
F Soriano et al.
The Journal of antimicrobial chemotherapy, 38(2), 227-236 (1996-08-01)
In an attempt to determine the susceptibility breakpoints for amoxycillin, co-amoxiclav and cefotaxime in pneumococcal pneumonia, a neutropenic mouse model was established and tested with two strains having different susceptibility to penicillins and cefotaxime. With a penicillin-sensitive strain (MIC/MBC =
Martijn F Schenk et al.
PLoS genetics, 8(6), e1002783-e1002783 (2012-07-05)
For a quantitative understanding of the process of adaptation, we need to understand its "raw material," that is, the frequency and fitness effects of beneficial mutations. At present, most empirical evidence suggests an exponential distribution of fitness effects of beneficial
Hetty Blaak et al.
International journal of food microbiology, 168-169, 8-16 (2013-11-12)
The attribution of fresh produce to the overall community-associated exposure of humans to ESBL- or AmpC-producing bacteria is currently unknown. To address this issue, the prevalence of ESBL- and AmpC-producing Enterobacteriaceae on fresh produce produced in the Netherlands was determined.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務