推薦產品
生物源
rabbit
品質等級
共軛
unconjugated
抗體表格
IgG fraction of antiserum
抗體產品種類
primary antibodies
無性繁殖
polyclonal
形狀
PBS solution
分子量
antigen 42 kDa
物種活性
human, mouse
技術
microarray: suitable
western blot: 1:3,000 using whole cell extract of the human endothelial ECV304 cell line.
UniProt登錄號
運輸包裝
dry ice
儲存溫度
−20°C
目標翻譯後修改
unmodified
基因資訊
human ... PARVA(55742)
mouse ... Parva(57342)
一般說明
Actopaxin is a member of actin binding proteins that include beta-parvin/affixin and gamma-parvin. Actopaxin is known to associate with F-actin and paxillin LD motifs LD1 and LD4. This protein also directly binds to serine/threonine integrin-linked kinase (ILK). Studies have reported that actopaxin may be involved in integrin-dependent remodeling of the actin cytoskeleton during cell adhesion, motility and division.
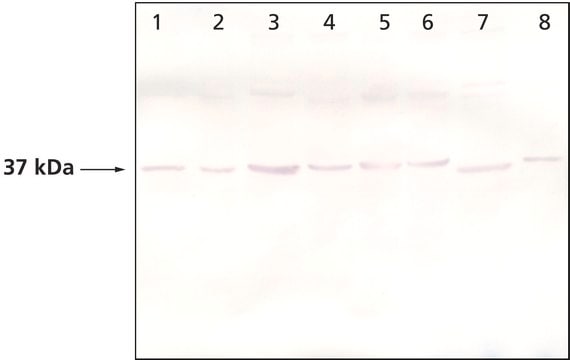
Rabbit anti-actopaxin recognizes actopaxin (42 kDa). Staining of actopaxin in immunoblotting is specifically inhibited with actopaxin immunizing peptide (mouse, amino acids 35-53).
Rabbit anti-actopaxin recognizes actopaxin (42 kDa). Staining of actopaxin in immunoblotting is specifically inhibited with actopaxin immunizing peptide (mouse, amino acids 35-53).
免疫原
synthetic peptide corresponding to the N-terminal region of mouse actopaxin (amino acids 35-53). The sequence is identical in human actopaxin and has considerable homology (~70%) with β-parvin/affixin, but no homology with γ-parvin.
應用
Actopaxin was detected in HEK 293 cell lysates at 42 kDa by western blot using rabbit anti-actopaxin antibody at a 1:1000 dilution. In these cells, actopaxin associated with an ILK complex.
Applications in which this antibody has been used successfully, and the associated peer-reviewed papers, are given below.
Immunofluorescence (1 paper)
Immunofluorescence (1 paper)
Rabbit anti-actopaxin can also be used for protein microaarays.
外觀
Solution in 0.01 M phosphate buffered saline, pH 7.4, containing 15 mM sodium azide
免責聲明
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
未找到適合的產品?
試用我們的產品選擇工具.
S Yamaji et al.
The Journal of cell biology, 153(6), 1251-1264 (2001-06-13)
Focal adhesions (FAs) are essential structures for cell adhesion, migration, and morphogenesis. Integrin-linked kinase (ILK), which is capable of interacting with the cytoplasmic domain of beta1 integrin, seems to be a key component of FAs, but its exact role in
Thitima Keskanokwong et al.
The Journal of biological chemistry, 282(32), 23205-23218 (2007-06-08)
Kidney anion exchanger 1 (kAE1) mediates chloride/bicarbonate exchange at the basolateral membrane of kidney alpha-intercalated cells, thereby facilitating bicarbonate reabsorption into the blood. Human kAE1 lacks the N-terminal 65 residues of the erythroid form (AE1, band 3), which are essential
Sotiris N Nikolopoulos et al.
The Journal of biological chemistry, 277(2), 1568-1575 (2001-11-06)
Paxillin is a focal adhesion adapter protein involved in integrin signaling. We have recently reported that the paxillin LD1 motif acts as a binding interface for both the actin-binding protein actopaxin and the serine/threonine integrin-linked kinase (ILK). In this report
Iveta Dobreva et al.
Journal of proteome research, 7(4), 1740-1749 (2008-03-11)
Protein-protein interactions play an essential role in the regulation of vital biological functions. Through a network of interactions, integrin-linked kinase (ILK) functions downstream of integrin receptors to control cell spreading, migration, growth, survival, and cell cycle progression. Despite many reports
T M Olski et al.
Journal of cell science, 114(Pt 3), 525-538 (2001-02-15)
We have identified and cloned a novel 42-kDa protein termed alpha-parvin, which has a single alpha-actinin-like actin-binding domain. Unlike other members of the alpha-actinin superfamily, which are large multidomain proteins, alpha-parvin lacks a rod domain or any other C-terminal structural
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務