推薦產品
等級
pharmaceutical primary standard
API 家族
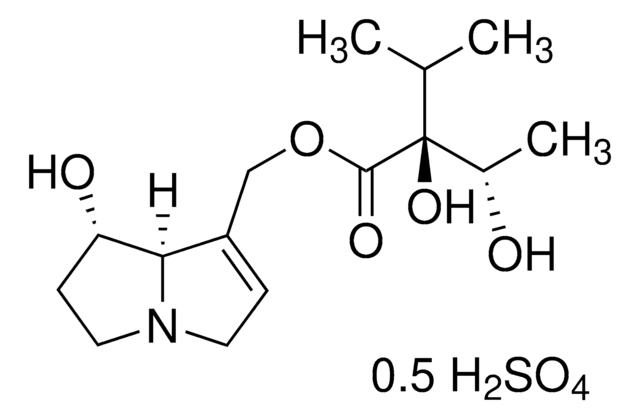
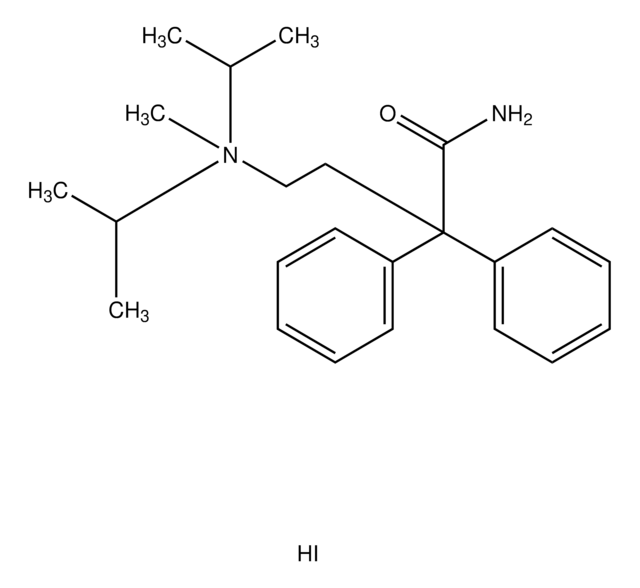
thioridazine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
InChI
1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3
InChI 密鑰
KLBQZWRITKRQQV-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Thioridazine for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Eduardo Abbate et al.
The Journal of antimicrobial chemotherapy, 67(2), 473-477 (2011-12-03)
Current drug choices to treat extensively drug-resistant (XDR) tuberculosis (TB) are scarce; therefore, information on the safety, tolerability and efficacy of alternative regimens is of utmost importance. The aim of this study was to describe the management, drug adverse effects
Maija Purhonen et al.
Pharmacoepidemiology and drug safety, 21(11), 1227-1231 (2012-09-04)
Thioridazine is a first-generation antipsychotic drug that was withdrawn from the market worldwide in 2005. The outcome of clinically stable schizophrenia patients who used thioridazine before market withdrawal was evaluated. Nationwide registers in Finland were utilized to study thioridazine use
Marianne Ø Poulsen et al.
Research in microbiology, 164(2), 181-188 (2012-10-24)
The neuroleptic antipsychotic derivate thioridazine has been shown to increase the susceptibility of a methicillin-resistant Staphylococcus aureus (MRSA) isolate towards dicloxacillin. The aim of this study was to investigate the combinatorial effect of the two drugs on a broad selection
John Bola et al.
The Cochrane database of systematic reviews, (6)(6), CD006374-CD006374 (2011-06-17)
Long-term treatment with antipsychotic medications in early episode schizophrenia spectrum disorders is common, but both short and long-term effects on the illness are unclear. There have been numerous suggestions that people with early episodes of schizophrenia appear to respond differently
Jørn B Christensen et al.
PloS one, 8(3), e57493-e57493 (2013-03-19)
A long list of chemotherapeutical drugs used in the treatment of the peripheral and the central nervous systems possess anti-microbial activity. Some of these neurotropic compounds are chiral, with the one stereo isomeric form exaggerating reduced neurotropism. This is the
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務