推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. Y0001561
traceable to USP 1000408
API 家族
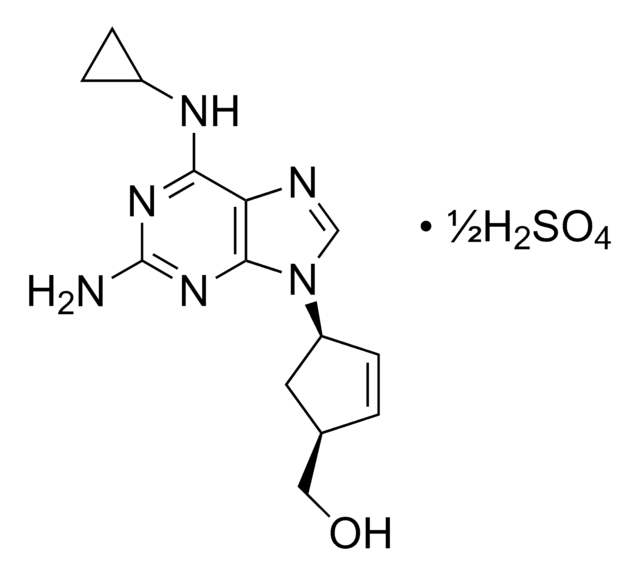
abacavir
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-30°C
InChI
1S/2C14H18N6O.H2O4S/c2*15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21;1-5(2,3)4/h2*1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19);(H2,1,2,3,4)/t2*8-,10+;/m11./s1
InChI 密鑰
WMHSRBZIJNQHKT-FFKFEZPRSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
應用
分析報告
其他說明
腳註
相關產品
訊號詞
Warning
危險分類
Carc. 2 - Eye Irrit. 2 - Muta. 2 - Repr. 2 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
文章
Analysis of 20 pharmaceutical compounds in calf serum using Supel™ Swift HLB SPE cartridge for cleanup and LC-MS determination and another commercially available HLB cartridge for comparison.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務