推薦產品
等級
pharmaceutical primary standard
API 家族
ifosfamide
製造商/商標名
EDQM
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
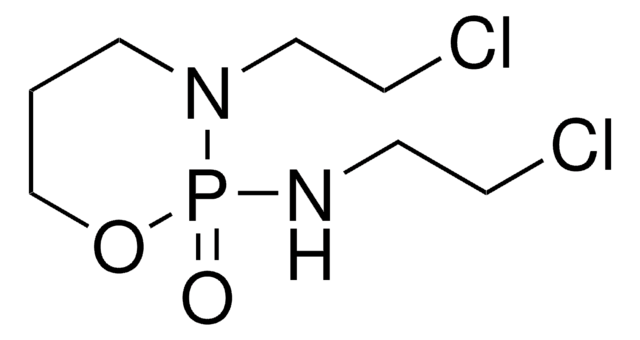
ClCCNP1(=O)OCCCN1CCCl
InChI
1S/C7H15Cl2N2O2P/c8-2-4-10-14(12)11(6-3-9)5-1-7-13-14/h1-7H2,(H,10,12)
InChI 密鑰
HOMGKSMUEGBAAB-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Ifosfamide EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
异环磷酰胺是一种氮芥类化合物,是环磷酰胺的结构异构体。 异环磷酰胺是一种前药,必须通过细胞色素P450转化为生物活性成分。 它在癌症化疗中可用作抗肿瘤药,但是异环磷酰胺比环磷酰胺更可能引起肾毒性。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Carc. 1B - Eye Irrit. 2 - Muta. 1B - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
T Ajithkumar et al.
Clinical oncology (Royal College of Radiologists (Great Britain)), 19(2), 108-114 (2007-03-16)
Encephalopathy is a potentially fatal toxicity of ifosfamide. Clinical manifestations of encephalopathy range from fatigue and confusion to coma and death. Early identification of this toxicity and prompt cessation of ifosfamide are the essential elements in the management of ifosfamide
Jean-Yves Blay et al.
European journal of cancer (Oxford, England : 1990), 50(6), 1137-1147 (2014-02-12)
This randomised phase III trial evaluated first-line trabectedin versus doxorubicin-based chemotherapy (DXCT) in patients with advanced/metastatic translocation-related sarcomas (TRS). Patients were randomly assigned (1:1) to receive trabectedin 1.5mg/m2 24-h intravenous (i.v.) infusion every 3 weeks (q3wk) (Arm A), or doxorubicin
Renée L Mulder et al.
The Cochrane database of systematic reviews, (2)(2), CD006300-CD006300 (2010-02-19)
Alkylating agents, such as cyclophosphamide and ifosfamide, play a major role in the improved survival of children and young adults with bone and soft tissue sarcoma. However, there is still controversy as to their comparative anti-tumour efficacy and possible adverse
Metin Tascilar et al.
The oncologist, 12(11), 1351-1360 (2007-12-07)
The treatment outcome of patients with locally advanced and metastatic soft tissue sarcomas is poor. Doxorubicin is regarded as standard treatment, but its use is featured by the occurrence of cardiotoxicity. This hinders the administration of this drug at high
Priti N Patel
The Annals of pharmacotherapy, 40(2), 299-303 (2006-01-05)
To evaluate the use of methylene blue for the treatment of ifosfamide-induced encephalopathy. MEDLINE (1966-August 2005) and International Pharmaceutical Abstracts (1971-August 2005) were searched using the terms methylene blue, ifosfamide, encephalopathy, and neurotoxicity. Several case reports and one retrospective chart
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務