全部照片(1)
About This Item
經驗公式(希爾表示法):
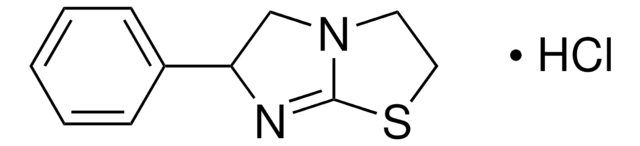
C11H12N2S · HCl
CAS號碼:
分子量::
240.75
MDL號碼:
分類程式碼代碼:
41116107
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
levamisole
形狀
powder
品質
British Pharmacopoeia (BP) Reference Standard
儲存期限
limited shelf life, expiry date on the label
製造商/商標名
BP
mp
230-233 °C (lit.)
應用
pharmaceutical
pharmaceutical small molecule
格式
neat
儲存溫度
2-8°C
SMILES 字串
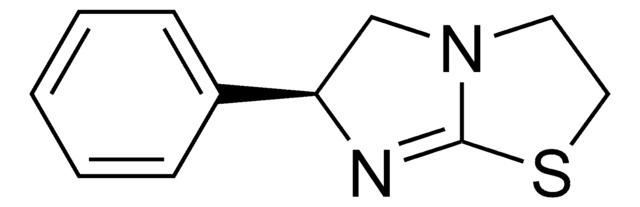
Cl.C1CN2C[C@@H](N=C2S1)c3ccccc3
InChI
1S/C11H12N2S.ClH/c1-2-4-9(5-3-1)10-8-13-6-7-14-11(13)12-10;/h1-5,10H,6-8H2;1H/t10-;/m1./s1
InChI 密鑰
LAZPBGZRMVRFKY-HNCPQSOCSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Levamisole hydrochloride BP Reference standard, intended for use in laboratory tests only as specifically prescribed in the British Pharmacopoeia.
Also used in monographs such as:Levamisole Injection Levamisole Oral Solution
Also used in monographs such as:
包裝
Unit quantity: 100 mg. Subject to change. The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity please visit British Pharmacopoeia
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務