推薦產品
化驗
≥97.0% (GC)
折射率
n20/D 1.530
密度
1.215 g/mL at 20 °C (lit.)
官能基
tosylate
儲存溫度
2-8°C
SMILES 字串
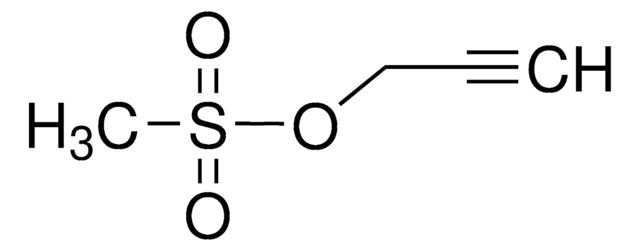
Cc1ccc(cc1)S(=O)(=O)OCC#C
InChI
1S/C10H10O3S/c1-3-8-13-14(11,12)10-6-4-9(2)5-7-10/h1,4-7H,8H2,2H3
InChI 密鑰
LMBVCSFXFFROTA-UHFFFAOYSA-N
應用
Propargyl p-toluenesulfonate can be used as an initiator in the synthesis of linear and cyclic poly(2-isopropyl-2-oxazoline)s by cationic ring-opening polymerization of 2-isopropyl-2-oxazoline.[1]
It can also be used as a reagent to synthesize:
It can also be used as a reagent to synthesize:
- 2-hydroxy-4-pentynoic acid by an alkylation reaction with diethyl 2-acetamidomalonate followed by subsequent hydrolysis, decarboxylation, diazotization, and hydroxylation reactions.[2]
- Furan derivatives by Pd-catalyzed reaction with acylchromates.[3]
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
212.0 °F - closed cup
閃點(°C)
100 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
客戶也查看了
Preparation of furans by palladium-catalyzed reaction of acylchromates and propargylic tosylates
Nakamura M, et al.
Heterocycles, 59(1), 333-345 (2003)
Linear and cyclic poly (2-isopropyl-2-oxazoline) s for fine control of thermoresponsiveness
Jung Yongseok, et al.
European Polymer Journal, 88, 605-612 (2017)
An economical and safe procedure to synthesize 2-hydroxy-4-pentynoic acid: A precursor towards `clickable?biodegradable polylactide
Zhang Quanxuan, et al.
Beilstein Journal of Organic Chemistry, 10(1), 1365-1371 (2014)
Lucca Trachsel et al.
ACS nano, 14(8), 10054-10067 (2020-07-07)
The physicochemical properties of cyclic polymer adsorbates are significantly influenced by the steric and conformational constraints introduced during their cyclization. These translate into a marked difference in interfacial properties between cyclic polymers and their linear counterparts when they are grafted
Yuji Odagaki et al.
Journal of pharmacological sciences, 125(2), 157-168 (2014-05-23)
The functional activation of Gi/o proteins coupled to muscarinic acetylcholine receptors (mAChRs) was investigated with the conventional guanosine-5'-O-(3-[(35)S]thio) triphosphate ([(35)S]GTPγS) binding assay in rat brain membranes. The most efficacious stimulation elicited by acetylcholine or carbachol (CCh) was obtained in striatal
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務