推薦產品
描述
Flash point (deg F): >230
形狀
liquid
折射率
n/D 1.487
密度
1.128 at 25 °C
儲存溫度
−20°C
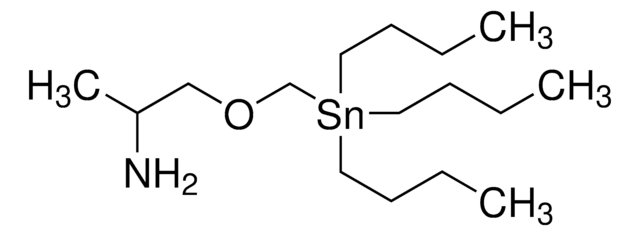
SMILES 字串
CCCC[Sn](CCCC)(COC(C)CN)CCCC
InChI
1S/C4H10NO.3C4H9.Sn/c1-4(3-5)6-2;3*1-3-4-2;/h4H,2-3,5H2,1H3;3*1,3-4H2,2H3;
InChI 密鑰
MALMPZOIHFDDCK-UHFFFAOYSA-N
應用
SnAP Reagents provide a one-step route, in tandem with various aldehyde substrates, to saturated N-heterocycles. The synthesis of N-heterocycles through SnAP Reagents requires mild reaction conditions, and aldehydes bearing aryl, heteroaryl, glyoxyl, aliphatic, and halogenated groups are well tolerated. This product was introduced in collaboration with the Bode Research Group
Automate your N-heterocycle formation with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your N-heterocycle formation with Synple Automated Synthesis Platform (SYNPLE-SC002)
其他說明
Technology spotlight: SnAP Reagents
Professor product portal: Jeffrey Bode Research Group
SnAP Reagents for the Synthesis of Piperazines and Morpholines
SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes
SnAP Reagents for a Cross-Coupling Approach to the One-Step Synthesis of Saturated N-Heterocycles
Professor product portal: Jeffrey Bode Research Group
SnAP Reagents for the Synthesis of Piperazines and Morpholines
SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes
SnAP Reagents for a Cross-Coupling Approach to the One-Step Synthesis of Saturated N-Heterocycles
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Woon-Yew Siau et al.
Journal of the American Chemical Society, 136(51), 17726-17729 (2014-12-09)
The combination of cyclic ketones and stannyl amine protocol (SnAP) reagents affords saturated, spirocyclic N-heterocycles under operationally simple reaction conditions. The resulting, N-unprotected spirocyclic amines are in great demand as scaffolds for drug discovery and development. The union of SnAP
Cam-Van T Vo et al.
Nature chemistry, 6(4), 310-314 (2014-03-22)
Interest in saturated N-heterocycles as scaffolds for the synthesis of bioactive molecules is increasing. Reliable and predictable synthetic methods for the preparation of these compounds, especially medium-sized rings, are limited. We describe the development of SnAP (Sn amino protocol) reagents
Michael U Luescher et al.
Organic letters, 16(4), 1236-1239 (2014-02-08)
Substituted piperazines and morpholines are valuable structural motifs in biologically active compounds, but are not easily prepared by contemporary cross-coupling approaches. In this report, we introduce SnAP reagents for the transformation of aldehydes into N-unprotected piperazines and morpholines. This approach
SnAP reagents for the transformation of aldehydes into substituted thiomorpholines--an alternative to cross-coupling with saturated heterocycles.
Cam-Van T Vo et al.
Angewandte Chemie (International ed. in English), 52(6), 1705-1708 (2013-01-03)
條款
SnAP Reagents facilitate synthesis of saturated N-heterocycles for diverse structures.
SnAP 試劑有助於合成各種結構的飽和 N-heterocycles 。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務