全部照片(1)
About This Item
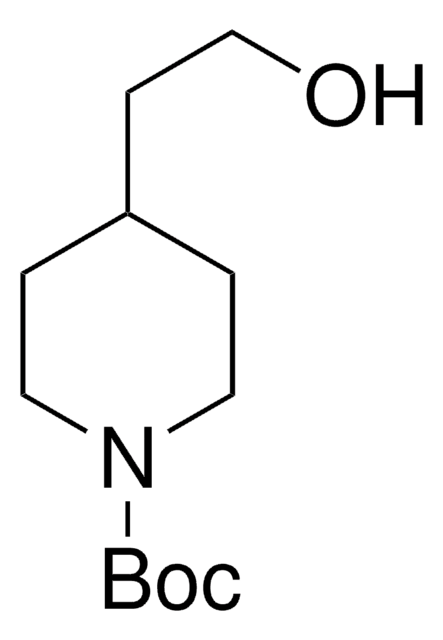
經驗公式(希爾表示法):
C11H21NO3
CAS號碼:
分子量::
215.29
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
暫時無法取得訂價和供貨情況
推薦產品
化驗
97%
形狀
solid
mp
78-82 °C (lit.)
官能基
hydroxyl
SMILES 字串
CC(C)(C)OC(=O)N1CCC(CO)CC1
InChI
1S/C11H21NO3/c1-11(2,3)15-10(14)12-6-4-9(8-13)5-7-12/h9,13H,4-8H2,1-3H3
InChI 密鑰
CTEDVGRUGMPBHE-UHFFFAOYSA-N
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Synthesis and nanostructures of 5, 10, 15, 20-tetrakis (4-piperidyl) porphyrin.
JacobsenJL, et al.
Tetrahedron, 69(48), 10507-10515 (2013)
Fluorine-18 labeling of 6, 7-disubstituted anilinoquinazoline derivatives for positron emission tomography (PET) imaging of tyrosine kinase receptors: synthesis of 18F-Iressa and related molecular probes.
Seimbille Y, et al.
Journal of Labelled Compounds & Radiopharmaceuticals, 48(11), 829-843 (2005)
Hideki Kubota et al.
Bioorganic & medicinal chemistry letters, 14(12), 3049-3052 (2004-05-20)
A series of piperidinoalkanoyl-1,2,3,4-tetrahydroisoquinoline derivatives were synthesized, and their bradycardic activities were investigated in the isolated right atria of guinea pigs and in conscious rats. These efforts identified the achiral compound 2f, which exhibited potent and long-lasting bradycardic activity with
Daniele Zampieri et al.
European journal of medicinal chemistry, 44(1), 124-130 (2008-04-29)
We describe here the synthesis and the binding interaction with sigma(1) and sigma(2) receptors of a series of new benzo[d]oxazol-2(3H)-one derivatives variously substituted on the N-benzyl moiety. The results of binding studies confirm the notion that the benzoxazolone moiety confers
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務