推薦產品
化驗
97%
mp
59-62 °C (lit.)
官能基
carboxylic acid
ketone
SMILES 字串
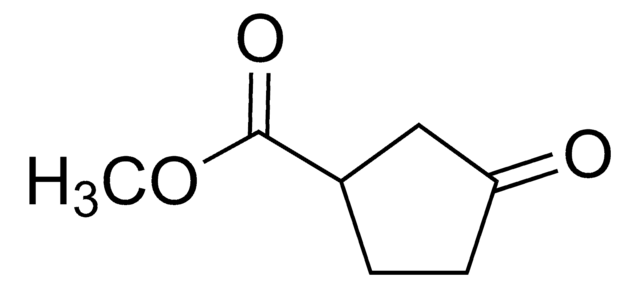
OC(=O)C1CCC(=O)C1
InChI
1S/C6H8O3/c7-5-2-1-4(3-5)6(8)9/h4H,1-3H2,(H,8,9)
InChI 密鑰
RDSNBKRWKBMPOP-UHFFFAOYSA-N
一般說明
3-Oxo-1-cyclopentanecarboxylic acid , also known as 3-oxocyclopentanecarboxylic acid, is a keto acid derivative. It undergoes Curtius rearrangement with diphenyl phosphoryl azide and triethylamine in tert-butanol to form the corresponding boc-protected 1-(3-oxo)urea derivative.[1]
應用
使用细胞色素 P450 BM-3 突变体的研究生物羟基化反应的底物。[2]
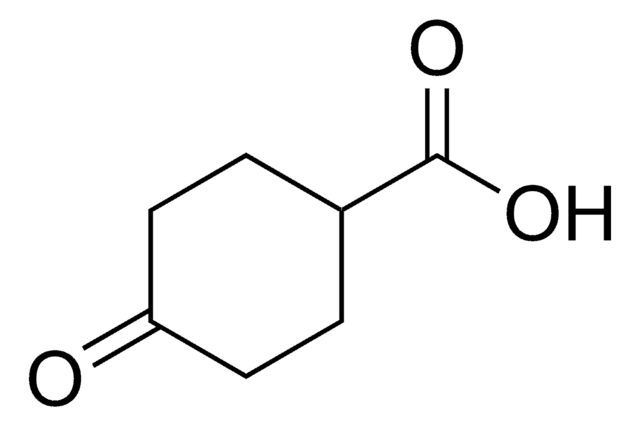
3-Oxo-1-cyclopentanecarboxylic acid may be used in the preparation of 3-hydroxycyclopentanecarboxylic acid via hydrogenation.[3]
法律資訊
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Studies of Configuration. V. The Preparation and Configuration of cis-3-Methoxycyclopentanecarboxylic Acid.
Noyce D and Fessenden J.
The Journal of Organic Chemistry, 24(5), 715-717 (1959)
Dieter F Münzer et al.
Chemical communications (Cambridge, England), (20), 2597-2599 (2005-05-19)
Substrate engineered, achiral carboxylic acid derivative was biohydroxylated with various mutants of cytochrome P450 BM-3 to give two out of the four possible diastereoisomers in high de and ee. The BM-3 mutants exhibit up to 9200 total turnovers for hydroxylation
Boc-protected 1-(3-oxocycloalkyl) ureas via a one-step Curtius rearrangement: mechanism and scope.
Sun X, et al.
Tetrahedron Letters, 55(4), 842-844 (2014)
文章
Phase I biotransformation reactions increase drug compound polarity, mainly occurring in hepatic circulation.
第一階段生物轉化反應會增加藥物化合物的極性,主要發生在肝循環中。
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務