推薦產品
化驗
97%
mp
43-48 °C (lit.)
儲存溫度
2-8°C
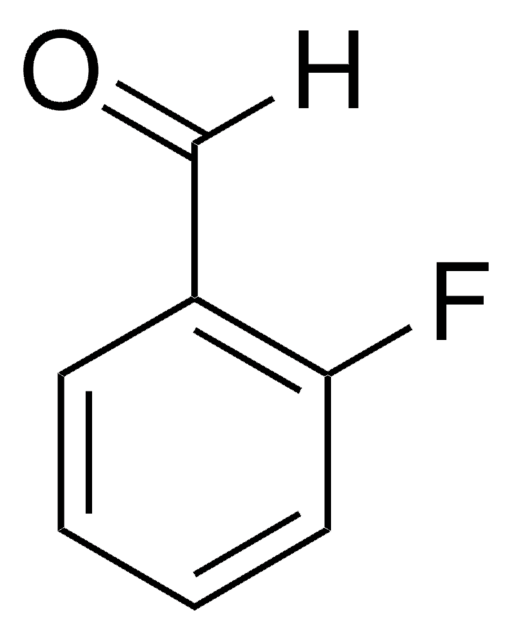
SMILES 字串
COc1ccc(C=O)c(F)c1
InChI
1S/C8H7FO2/c1-11-7-3-2-6(5-10)8(9)4-7/h2-5H,1H3
InChI 密鑰
UNWQNFJBBWXFBG-UHFFFAOYSA-N
一般說明
2-Fluoro-4-methoxybenzaldehyde is a fluorinated aromatic aldehyde. It can be prepared from 4-bromo-3-fluoroanisole.[1]
應用
2-Fluoro-4-methoxybenzaldehyde may be used in the preparation of:
- fluorine containing 2,4,5-trisubstituted imidazole[2]
- 1-(2-fluoro-4-methoxyphenyl)-2-propanone[3]
- 6-(2-fluoro-4-methoxyphenyl)fulvene[4]
- 10-(2-fluoro-4-methoxyphenyl)-6,7,9,10-tetrahydro-1Hfuro[3,4-b]pyrazolo[3,4-f]quinolin-9-one[5]
- polyhydroquinoline (PHQ)[6]
- 3-(2-fluoro-4-methoxyphenyl) acrylic acid methyl ester[7]
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Indium trifluoride: A highly efficient catalyst for the synthesis of fluorine-containing 2, 4, 5-trisubstituted imidazoles under solvent-free conditions.
Reddy MV and Jeong YT.
Journal of Fluorine Chemistry, 142, 45-51 (2012)
Polystyrene-Supported p-Toluenesulfonic Acid: A New, Highly Efficient, and Recyclable Catalyst for the Synthesis of Hydropyridine Derivatives under Solvent-Free Conditions.
Reddy MV and Jeong YT.
Synlett, 23(20), 2985-2991 (2012)
Fluorinated derivatives of titanocene Y: synthesis and cytotoxicity studies.
Claffey J, et al.
European Journal of Organic Chemistry, 26, 4074-4082 (2008)
Synthesis, cytotoxic activity and docking studies of new 4-aza-podophyllotoxin derivatives.
Hatti I, et al.
Medicinal Chemistry Research, 24(8), 3305-3313 (2015)
Jack G Parsons et al.
Molecules (Basel, Switzerland), 9(6), 449-458 (2007-11-17)
The synthesis of (2S)-2-benzyloxymethyl-3-(2-fluoro-4-methoxyphenyl)- propionic acid, (2S)-2-benzyloxymethyl-3-(2-fluoro-4-methylphenyl)propionic acid and (2S)-2-benzyl-oxymethyl-3-(2,4-dimethylphenyl)propionic acid has been achieved by TiCl4 mediated alkylation of the corresponding (4R)-4-benzyl-3-[3-(2-fluoro-4-methoxyphenyl-, 2-fluoro-4-methylphenyl-, 2,4- dimethylphenyl-)propionyl]-2-oxazolidinones, followed by hydrolysis of the chiral auxiliary. The stereochemistry of the alkylation reaction was confirmed
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務