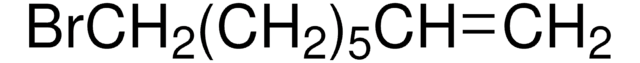推薦產品
化驗
≥97%
折射率
n20/D 1.466 (lit.)
bp
27 °C/1 mmHg (lit.)
密度
1.162 g/mL at 25 °C (lit.)
官能基
alkyl halide
allyl
bromo
SMILES 字串
BrCCCCCC=C
InChI
1S/C7H13Br/c1-2-3-4-5-6-7-8/h2H,1,3-7H2
InChI 密鑰
GNYDYUQVALBGGZ-UHFFFAOYSA-N
一般說明
7-Bromo-1-heptene is a linear halogenated alkene.
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
136.9 °F - closed cup
閃點(°C)
58.3 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
客戶也查看了
Alan P Kozikowski et al.
ChemMedChem, 4(7), 1095-1105 (2009-04-28)
A series of benzolactam compounds were synthesized, some of which caused a concentration-dependent increase in sAPPalpha and decrease in Abeta production in the concentration range of 0.1-10 microM. Moreover, some compounds showed neuroprotective effects in the 10-20 microM range in
Norikazu Nishino et al.
Bioorganic & medicinal chemistry, 16(1), 437-445 (2007-09-29)
Inhibitors of histone deacetylases (HDACs) are a promising class of anticancer agents that effect gene regulation. To know the interaction of aliphatic cap groups with HDACs, cyclic tetrapeptide and bicyclic peptide disulfide hybrids were synthesized without aromatic ring in their
G H Stoll et al.
Journal of lipid research, 32(5), 843-857 (1991-05-01)
An analogue of the long-chain fatty acid salt, sodium stearate, was synthesized in which the hydrogen atoms at carbons 2, 3, and 18 were replaced by fluorine. The key step in the synthesis was the addition of 3-iodo-2,2,3,3-tetrafluoropropanoic acid amide
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務