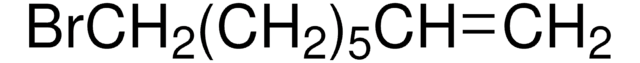推薦產品
化驗
95%
形狀
liquid
折射率
n20/D 1.463 (lit.)
bp
126-127 °C/765 mmHg (lit.)
密度
1.258 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
BrCCCC=C
InChI
1S/C5H9Br/c1-2-3-4-5-6/h2H,1,3-5H2
InChI 密鑰
LPNANKDXVBMDKE-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
5-溴-1-戊烯用于立体选择性合成 7α-(3-羧丙基) 雌二醇。用于制备具有硫代糖苷键的唾液酸的硫代乙酸酯 11。它还被用作最近合成 DL-组织毒素和含二苯甲酮的脂肪酸的起始材料。
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
87.8 °F - closed cup
閃點(°C)
31 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Jun-Ichi Sakamoto et al.
Bioorganic & medicinal chemistry letters, 17(3), 717-721 (2006-11-11)
An efficient synthesis of a series of carbosilane dendrimers uniformly functionalized with alpha-thioglycoside of sialic acid was accomplished. The results of a preliminary study on biological responses against influenza virus sialidases using thiosialoside clusters showed that some of the glycodendrimers
Maheswaran S Karatholuvhu et al.
Journal of the American Chemical Society, 128(39), 12656-12657 (2006-09-28)
The synthesis of (+/-)-histrionicotoxin has been achieved in just nine steps using a two-directional synthesis strategy. Key reactions include a two-directional cross-metathesis, a tandem oxime formation/Michael addition/1,4-prototopic shift/[3 + 2]-cycloaddition cascade, a selective Z,Z-bisenyne formation, and a one-pot N-O and
Yifan Dong et al.
Carbohydrate polymers, 221, 37-47 (2019-06-23)
Bile salts tend to form micelles in aqueous media and can thereby contribute to drug solubilization; they also exhibit crystallization inhibition properties that can stabilize supersaturated drug solutions. Herein, we explore conjugation of bile salts with polysaccharides to create new
Yonghong Gan et al.
The Journal of organic chemistry, 71(25), 9487-9490 (2006-12-02)
Syntheses of new benzophenone-containing fatty acids (FABPs) 1, 5, and 6 and a new route to FABP 3 are described. Combined with the known 2 and 4, these FABPs comprise a set of photoactivatable fatty acid analogues with the crosslinking
M Adamczyk et al.
Steroids, 62(12), 771-775 (1998-01-22)
Alkylation of 3,17 beta-bis(2-trimethylsilyl)ethoxymethyl-1,3,5(10) estratriene-6-one (2) with 5-bromo-1-pentene using NaHMDS in THF afforded 3,17 beta-bis(2-trimethylsilyl)ethoxymethyl-7-alpha-(4'pentenyl)-1,3,5(10) estratriene-6-one (3) in excellent stereoselectivity (> 95% epimeric excess). Functionalization of the side chain in compound 3 was accomplished via ozonolysis, oxidation and esterification to
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務