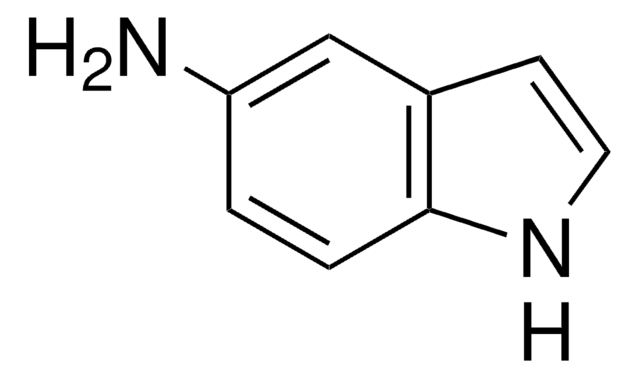
推薦產品
化驗
97%
mp
106-109 °C (lit.)
SMILES 字串
Nc1cccc2[nH]ccc12
InChI
1S/C8H8N2/c9-7-2-1-3-8-6(7)4-5-10-8/h1-5,10H,9H2
InChI 密鑰
LUNUNJFSHKSXGQ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
4-Aminoindole may be used to synthesize:
Reactant for preparation of:
- Inhibitors of bacterial thymidylate synthase
- Mimetics of non-alkaloid toxin lignan anticancer and antiviral agent Podophyllotoxin (PPT)
- Inhibitors of Gli1-mediated transcription in the Hedgehog pathway
- Protein kinase C θ (PKCθ) inhibitors
- Indolic non-peptidic HIV protease inhibitors
- Transient receptor potential cation channel subfamily V member 1 (TRPV1) antagonists
- Cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitors
- 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) inhibitors
- Short chain 4-substituted indoles as potent αvβ3 antagonist
- Ligands of serotonin transporter and 5-HT1A receptors
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
An efficient synthesis of azidoindoles and azidotryptophans.
Melhado LL and Leonard NJ.
The Journal of Organic Chemistry, 48(25), 5130-5133 (1983)
Synthestic approaches to the teleocidin-related tumour promoters: a total synthesis of (?)-indolactam V.
de Laszlo SE, et al.
Journal of the Chemical Society. Chemical Communications, 4, 344-346 (1986)
Maria Grazia Ferlin et al.
Journal of medicinal chemistry, 48(9), 3417-3427 (2005-04-29)
In our search for potential new anticancer drugs, we designed and synthesized a series of tricyclic compounds containing the antimitotic 2-phenylazaflavone chromophore fused to a pyrrole ring in a pyrroloquinoline structure. Compounds 8, 18, 19, 22, 23, 25 and 26
Cytokinin activity of azaindene, azanaphthalene, naphthalene, and indole derivatives.
Torigoe Y, et al.
Phytochemistry, 11(5), 1623-1630 (1972)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務