推薦產品
品質等級
化驗
98%
mp
100-103 °C (lit.)
官能基
aldehyde
SMILES 字串
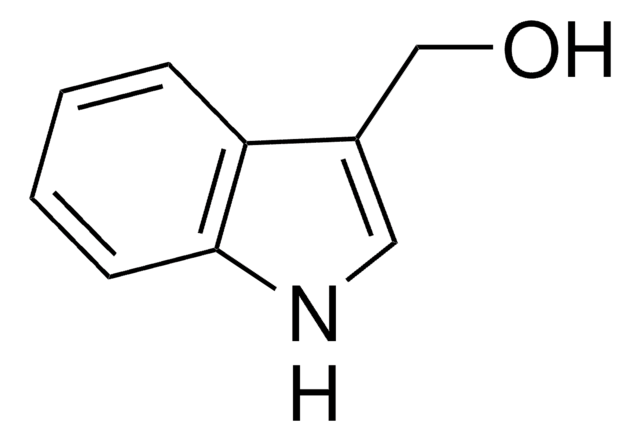
O=Cc1ccc2[nH]ccc2c1
InChI
1S/C9H7NO/c11-6-7-1-2-9-8(5-7)3-4-10-9/h1-6,10H
InChI 密鑰
ADZUEEUKBYCSEY-UHFFFAOYSA-N
應用
Indole-5-carboxaldehyde can be used as a reactant in the:
- Preparation of curcumin derivatives as anti-proliferative & anti-inflammatory agents[1]
- Preparation of analogs of botulinum neurotoxin serotype A protease inhibitors[2]
- Stereoselective synthesis of dibenzylideneacetone derivatives as β-amyloid imaging probes[3]
- Synthesis of para-para stilbenophanes by McMurry coupling[4]
- Stereoselective synthesis of heteroaromatic (E)-α,β-unsaturated ketones from aldehydes[5]
- Structure-based drug design of aurora kinase A inhibitors[6]
- Preparation of 5-indolyl linked 15- and 18-membered azacrown ethers to study their cation-π interactions.[7]
- Preparation of bibenzimidazole derivatives substituted 5-indolyl moiety in the study of inhibition of topoisomerase I activity.[8]
- Synthesis of (5-(4-(3,4,5-trimethoxybenzoyl)-1H-imidazol-2-yl)-1H-indol-2-yl)(3,4,5-trimethoxyphenyl)methanone[9] and radioiodinated indolochalcone.[10]
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Synthesis, QSAR and hypoglycemic activity of substituted 2,4-thiazolidinedione derivatives
Bahara, R. S.; Kulkarni, V. M.
Der Pharma Chemica, 3, 164-164 (2011)
S Jin et al.
Bioorganic & medicinal chemistry letters, 10(8), 719-723 (2000-04-27)
A series of 2'-heterocyclic derivatives of 5-phenyl-2,5'-1H-bibenzimidazoles were evaluated for topoisomerase I poisoning activity and cytotoxicity. Topo I poisoning activity was associated with 2'-derivatives that possessed a hydrogen atom capable of hydrogen bond formation, suggesting that the interatomic distances between
Todd J Eckroat et al.
Beilstein journal of organic chemistry, 9, 1012-1044 (2013-06-15)
The number of people suffering from Alzheimer's disease (AD) is expected to increase dramatically in the coming years, placing a huge burden on society. Current treatments for AD leave much to be desired, and numerous research efforts around the globe
Raquel Álvarez et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(12), 3406-3419 (2011-02-24)
The synthesis of a new family of methoxy-substituted [2.7]- and [2.8]paracyclophanes linked by 3-oxapentamethylene-1,5-dioxy and hexamethylene-1,6-dioxy bridges has been carried out by using the McMurry methodology. Related indole compounds were also synthesised. Olefin-to-diol ratios depended on the bridge length, the
Paul R Carlier et al.
The Journal of organic chemistry, 67(17), 6256-6259 (2002-08-17)
Tryptophan 1 (Trp) is superior to all other naturally occurring peptide residues in its ability to bind cations (the cation-pi interaction). In an effort to expand the toolbox of Trp-like amino acids, in this note we report catalytic asymmetric syntheses
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務