全部照片(2)
About This Item
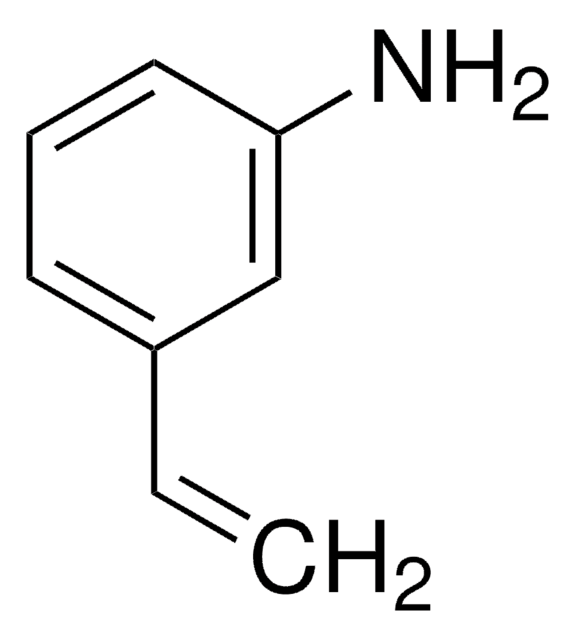
線性公式:
HC≡CC6H4NH2
CAS號碼:
分子量::
117.15
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
≥98%
反應適用性
reaction type: click chemistry
儲存溫度
2-8°C
SMILES 字串
Nc1cccc(c1)C#C
InChI
1S/C8H7N/c1-2-7-4-3-5-8(9)6-7/h1,3-6H,9H2
InChI 密鑰
NNKQLUVBPJEUOR-UHFFFAOYSA-N
一般說明
3-乙炔苯胺是一种末端炔烃,可以通过还原3-乙炔基硝基苯进行制备。
應用
3-乙炔苯胺可用于合成以下苯并恶嗪单体:
- 双{4-[3-(3-乙炔基苯基)(2H,4H-苯并[3,4-e]-1,3-恶嗪-6-基氧基)]苯基}苯基膦基-1-酮
- [3-(3-乙炔基苯基)(2H,4H-苯并[3,4-e]1,3-氧氮杂全氢化-6-基)][3-(3-乙炔基苯基)(2H,4HH-苯并[3,4-e]1,3恶嗪-6-基)]苯基膦基-1-酮
- 双[3-[3-(3-乙炔基苯基)(2H,4H-苯并[3,4-e]-1,3-恶嗪-6-基)氧基]苯基膦基-1-酮
- 合成琥珀酰(间乙炔基)二苯胺和癸二(间乙炔基)二苯胺。
- 作为多步合成盐酸厄洛替尼的试剂。
- 制备3-(3-乙炔基苯基)-6-甲基-2H, 4H-苯并[e]1,3-恶嗪。
- 制备3-[3-(4-乙酰氨基-苄基)-[1,2,4]恶二唑-5-基] -N-(3-乙炔基-苯基)-丙酰胺。
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
138.2 °F - closed cup
閃點(°C)
59 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Side-chain type benzoxazine-functional cellulose via click chemistry.
Agag T, et al.
Journal of Applied Polymer Science, 125(2), 1346-1351 (2012)
Paul L Chariou et al.
Nature nanotechnology, 14(7), 712-718 (2019-05-22)
Large doses of chemical pesticides are required to achieve effective concentrations in the rhizosphere, which results in the accumulation of harmful residues. Precision farming is needed to improve the efficacy of pesticides, but also to avoid environmental pollution, and slow-release
Qilin Mei et al.
Polymers, 12(5) (2020-05-03)
Benzoxazine resin has been paid more attention in the fields of aviation, electronics, automobiles and new energy industries because of its excellent comprehensive performance. Further application is limited, however, by shortcomings such as high brittleness and high curing temperature. Furthermore
Synthesis, analgesic and anti-inflammatory activities of novel 3-(4-acetamido-benzyl)-5-substituted-1,2,4-oxadiazoles.
Farooqui M, et al.
European Journal of Medicinal Chemistry, 44(2), 794-799 (2009)
Laura Marín-Caba et al.
Langmuir : the ACS journal of surfaces and colloids, 35(1), 203-211 (2018-12-24)
The design of versatile tools to improve cell targeting and drug delivery in medicine has become increasingly pertinent to nanobiotechnology. Biological and inorganic nanocarrier drug delivery systems are being explored, showing advantages and disadvantages in terms of cell targeting and
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務

![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![[3R(1′R,4R)]-(+)-4-乙酰氧基-3-[1-(叔丁基二甲基硅氧基)乙基]-2-氮杂环丁酮 98%](/deepweb/assets/sigmaaldrich/product/structures/346/391/4981e055-bdcd-454c-8c8f-519083b64769/640/4981e055-bdcd-454c-8c8f-519083b64769.png)







![2-[(三甲基硅基)乙炔基]苯胺 97%](/deepweb/assets/sigmaaldrich/product/structures/194/066/182b08e4-35d7-4b7b-8958-c917f64391fc/640/182b08e4-35d7-4b7b-8958-c917f64391fc.png)

![4[(三甲基硅基)乙炔基]苯胺 96%](/deepweb/assets/sigmaaldrich/product/structures/322/881/781cb36d-38c8-406f-afb2-1a4488f884e2/640/781cb36d-38c8-406f-afb2-1a4488f884e2.png)