推薦產品
化驗
99%
mp
86-89 °C (lit.)
官能基
carboxylic acid
SMILES 字串
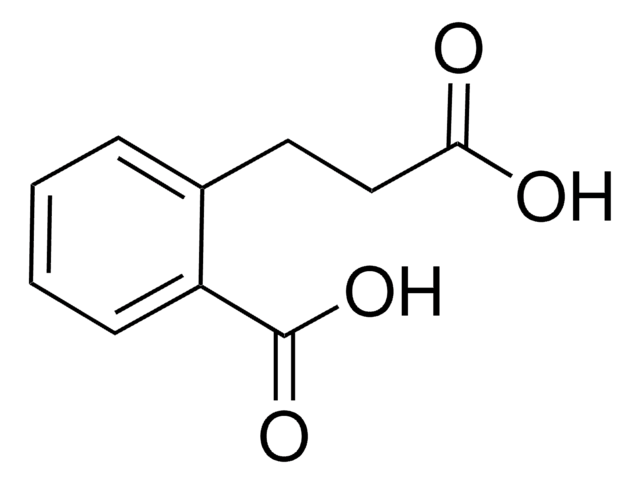
OC(=O)CCc1ccccc1O
InChI
1S/C9H10O3/c10-8-4-2-1-3-7(8)5-6-9(11)12/h1-4,10H,5-6H2,(H,11,12)
InChI 密鑰
CJBDUOMQLFKVQC-UHFFFAOYSA-N
一般說明
3-(2-Hydroxyphenyl) propionic acid is a phenyl propionic acid derivative. It was found to be one of the constituents of Justicia pectoralis Jacq. extract which was analyzed by gas chromatography/mass spectrometry (GC/MS).[1] The antiulcerogenic effect of 3-(2-hydroxyphenyl) propionic acid in the prevention of serotonin-induced ulcerogenesis has been studied in rats.[2] It has been reported to be one of the major microbial metabolite of both (+)-catechin and (-)-epicatechin by human faecal microbiota.[3] Crystal structure study reveals that 3-(2-hydroxyphenyl)propionic acid crystals are monoclinic with space group P21/c.[4]
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
R Burlingame et al.
Journal of bacteriology, 155(1), 113-121 (1983-07-01)
A number of laboratory strains and clinical isolates of Escherichia coli utilized several aromatic acids as sole sources of carbon for growth. E. coli K-12 used separate reactions to convert 3-phenylpropionic and 3-(3-hydroxyphenyl)propionic acids into 3-(2,3-dihydroxyphenyl)propionic acid which, after meta-fission
S Tanaka et al.
Planta medica, 55(3), 245-248 (1989-06-01)
Two active compounds that prevent serotonin-induced ulcerogenesis in rats were isolated from Chinese cinnamon (the stem bark of Cinnamomum cassia) and identified as 3-(2-hydroxyphenyl)-propanoic acid and its O-glucoside. The former compound, administered orally or parenterally to rats at a remarkably
J X de Vries et al.
Biomedical & environmental mass spectrometry, 15(8), 413-417 (1988-04-15)
The analysis of extracts from the South American plant Justicia pectoralis Jacq. permitted the identification, among other compounds, of coumarin, dihydrocoumarin, umbelliferone and 3-(2-hydroxyphenyl)propionic acid by gas chromatography/mass spectrometry (GC/MS); the acids and phenolic compounds were derivatized with diazomethane. GC/MS
Microbial metabolism of catechin stereoisomers by human faecal microbiota: comparison of targeted analysis and a non-targeted metabolomics method.
Aura AM, et al.
Phytochemistry Letters, 1(1), 18-22 (2008)
Structure of 3-(2-hydroxyphenyl) propionic acid.
Begum NS, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 48(6), 1076-1078 (1992)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務