推薦產品
化驗
97%
mp
85-89 °C (lit.)
官能基
carboxylic acid
hydroxyl
SMILES 字串
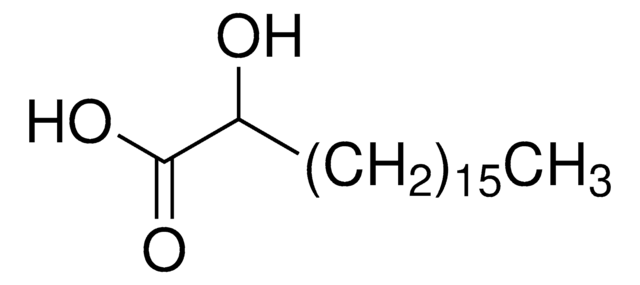
OCCCCCCCCCCCCCCC(O)=O
InChI
1S/C15H30O3/c16-14-12-10-8-6-4-2-1-3-5-7-9-11-13-15(17)18/h16H,1-14H2,(H,17,18)
InChI 密鑰
BZUNJUAMQZRJIP-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
15-Hydroxypentadecanoic acid is an ω-hydroxy acid. One of the method reported for its synthesis is from 1,12-dodecanolide.[1] It is reported to be one of the bioactive component in Tagetes erecta L. leaf and flower extract.[2]
15-Hydroxypentadecanoic acid undergoes lactonization reaction catalyzed by Mucor javanicus L46 and Mucor miehei to afford macrocyclic mono- and oligolactone derivatives.[3] Its lipase-catalyzed synthesis from 15-tetracosenoic acid in Malania Olcifera Chum oil has been proposed. It also participates in the biosynthesis of pentadecanolide.[4]
應用
15-Hydroxypentadecanoic acid is suitable reagent used in the following studies:
- As an internal standard in the quantification of formation of 11-hydroxylauric acid by gas chromatography.[5]
- In the synthesis of [16-14C]16DCA (DCA= dicarboxylic acid) by one-carbon elongation procedure at C15.[6]
- As an internal standard for the normalization of intensities in the mass spectra of plant cutin polymer.[7]
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Lipase catalyzed synthesis of pentadecanolide from 15-hydroxypentadecanoic acid.
Pan XB, et al.
Chinese Journal of Applied Chemistry / Ying Yong Hua Xue, 21(8), 850-852 (2004)
Zeolite-catalyzed macrolactonization of Ookoshi T and Onaka M. ω-hydroxyalkanoic acids in a highly concentrated solution.
Ookoshi T and Onaka M.
Tetrahedron Letters, 39(3), 293-296 (1998)
Enzymatic lactonization of 15-hydroxypentadecanoic and 16-hydroxyhexadecanoic acids to macrocyclic lactones.
Antczak U, et al.
Enzyme and Microbial Technology, 13(7), 589-593 (1991)
Sacha Ferdinandusse et al.
Journal of lipid research, 45(6), 1104-1111 (2004-04-03)
Dicarboxylic acids (DCAs) are omega-oxidation products of monocarboxylic acids. After activation by a dicarboxylyl-CoA synthetase, the dicarboxylyl-CoA esters are shortened via beta-oxidation. Although it has been studied extensively where this beta-oxidation process takes place, the intracellular site of DCA oxidation
Jamal Mustafa et al.
Lipids, 39(2), 167-172 (2004-05-12)
Derivatives of podophyllotoxin were prepared by coupling 10 FA with the C4-alpha-hydroxy function of podophyllotoxin. The coupling reactions between FA and podophyllotoxin were carried out by dicyclohexylcarbodiimide in the presence of a catalytic amount of dimethylaminopyridine to produce quantitative yields
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務

![2-[2-(2-甲氧基乙氧基)乙氧基]乙酸 technical grade](/deepweb/assets/sigmaaldrich/product/structures/335/694/b58c539b-141f-4ab2-98d9-5f46c748490b/640/b58c539b-141f-4ab2-98d9-5f46c748490b.png)