推薦產品
化驗
98%
形狀
liquid
折射率
n20/D 1.607 (lit.)
bp
123-125 °C/8 mmHg (lit.)
密度
1.387 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
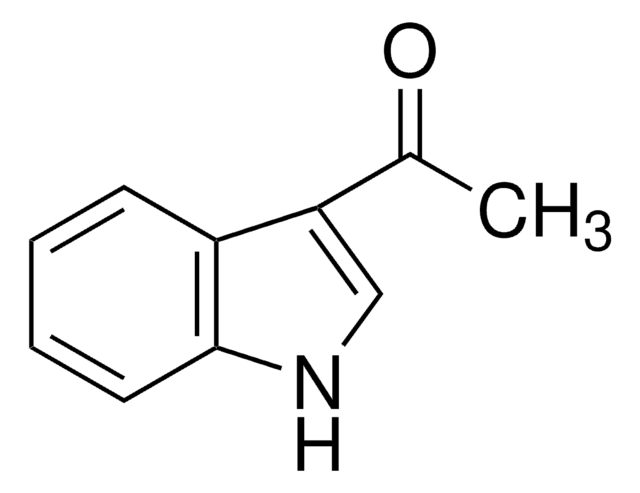
SMILES 字串
CC(=O)n1ccc2ccccc12
InChI
1S/C10H9NO/c1-8(12)11-7-6-9-4-2-3-5-10(9)11/h2-7H,1H3
InChI 密鑰
UUCUQJHYUPXDHN-UHFFFAOYSA-N
一般說明
Quantum chemical calculations of ground state energy, geometrical structure and vibrational wavenumbers of 1-acetylindole has been carried out using density functional (DFT/B3LYP) method.[1] Regioselective acylations of 1-acetylindole (N-acetylindole) under Friedel-Crafts reaction has been reported.[2] Reaction of 1-acetylindole with manganese(III) acetate in the presence of malonic acid, is reported to afford 4-acetyl-3,3a,4,8b-tetrahydro-2H-furo[3,2-b]indol-2-one.[3]
應用
1-Acetylindole may be used in the stereocontrolled synthesis of (±)-geissoschizine.[4] It may be used in the preparation of (1-acetyl-κO-indolyl-κC2)tetracarbonylmanganese, via a standard cyclomanganation procedure.[5]
Reactant for preparation of:
Reactant for:
- Antimycobacterial agents
- Cyclin-dependent kinase (CDK2) inhibitors
Reactant for:
- C3-C3 oxidative cross-coupling reactions
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
Mangenese (III) acetate oxidation of 1-acetylindole derivatives.
Izumi T, et al.
Journal of Heterocyclic Chemistry, 30(4), 1133-1136 (1993)
Synthesis and alkyne-coupling chemistry of cyclomanganated 1-and 3-acetylindoles, 3-formylindole and analogues.
Depree GJ, et al.
Journal of Organometallic Chemistry, 691(4), 667-679 (2006)
Vikas K Shukla et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 133, 626-638 (2014-07-06)
Quantum chemical calculations of ground state energy, geometrical structure and vibrational wavenumbers of 1-acetylindole were carried out using density functional (DFT/B3LYP) method with 6-311++G(d,p) basis set. The FT-IR and FT-Raman spectra were recorded in the condensed state. The fundamental vibrational
Regioselective acylations at the 2 and 6 position of N-acetylindole.
Cruz R, et al.
Tetrahedron Letters, 42(8), 1467-1469 (2001)
A concise, stereoselective synthesis of (?)-geissoschizine.
Bennasar M, et al.
Tetrahedron Letters, 37(50), 9105-9106 (1996)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務