全部照片(1)
About This Item
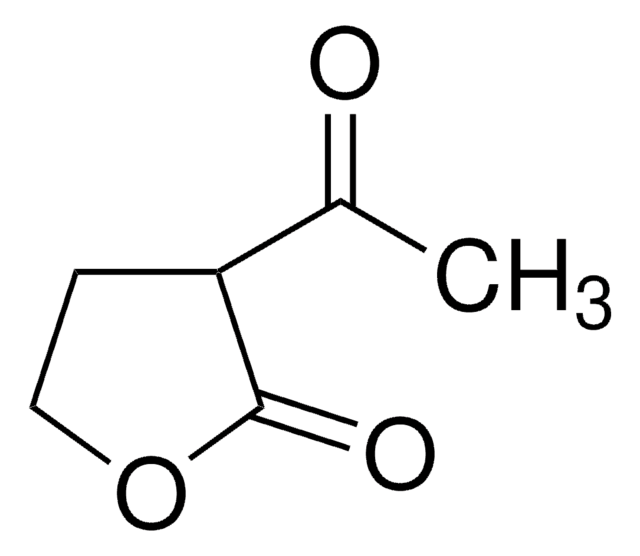
經驗公式(希爾表示法):
C4H6O3
CAS號碼:
分子量::
102.09
Beilstein:
80587
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
暫時無法取得訂價和供貨情況
推薦產品
等級
technical grade
品質等級
形狀
viscous liquid
折射率
n20/D 1.468 (lit.)
bp
133 °C/10 mmHg (lit.)
密度
1.309 g/mL at 25 °C (lit.)
官能基
ester
hydroxyl
SMILES 字串
OC1CCOC1=O
InChI
1S/C4H6O3/c5-3-1-2-7-4(3)6/h3,5H,1-2H2
InChI 密鑰
FWIBCWKHNZBDLS-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
α-Hydroxy-γ-butyrolactone is a 5-membered cyclic ester. It was obtained via tin-conversion of biomass-derived 1,3-dihydroxyacetone (DHA) and formaldehyde.[1]
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
客戶也查看了
Sho Yamaguchi et al.
Chemical communications (Cambridge, England), 50(35), 4600-4602 (2014-03-29)
The direct conversion of biomass-derived 1,3-dihydroxyacetone (DHA) and formaldehyde to α-hydroxy-γ-butyrolactone (HBL) was achieved through the use of tin(iv) chloride and a small amount of water and the yield reached up to 70%. The reaction mechanism was also investigated by
Efficient synthesis of enantiomerically pure (S)-d-azaproline starting from (R)-a-hydroxy-?-butyrolactone via the Mitsunobu reaction.
Voss E, et al.
Tetrahedron Asymmetry, 20(15), 1809-1812 (2009)
Natalia N Dioubankova et al.
Organic letters, 4(26), 4607-4610 (2002-12-20)
[reaction: see text] Two series of seco-pseudonucleoside synthons were synthesized from (R)-(+)-alpha-hydroxy-gamma-butyrolactone and (R)-(-)-pantolactone by aminolysis, side-chain protection, dimethoxytritylation, and phosphitylation or solid-phase attachment. The phosphoramidites and solid supports were used in automated DNA synthesis to prepare oligonucleotides modified with
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務