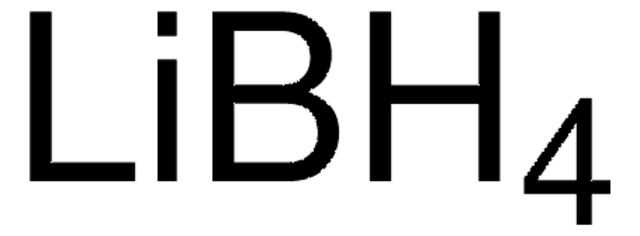If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdf
推薦產品
形狀
liquid
品質等級
反應適用性
reagent type: reductant
濃度
2.0 M in THF
密度
0.896 g/mL at 25 °C
SMILES 字串
[Li+].[H][B-]([H])([H])[H]
InChI
1S/BH4.Li/h1H4;/q-1;+1
InChI 密鑰
UUKMSDRCXNLYOO-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
- 镓、铟、铼和锌三(巯基咪唑基)氢硼酸配合物的制备
- 机械化学复分解反应
- 非催化水解制氢
- 大型金单层保护簇的生长
- 阴离子取代反应
- 脱氢反应
包裝
法律資訊
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
標靶器官
Central nervous system, Respiratory system
安全危害
儲存類別代碼
4.3 - Hazardous materials which set free flammable gases upon contact with water
水污染物質分類(WGK)
WGK 2
閃點(°F)
-0.4 °F - closed cup
閃點(°C)
-18 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
Helpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
-
Is the tetrahydrofuran (THF) used in Product 230200, Lithium Borohydride, stabilized?
1 answer-
The THF used in this product is stabilized with 0.025% butylated hydroxytoluene (BHT).
Helpful?
-
-
Is Product 230200, Lithium Borohydride, an energy carrier?
1 answer-
Lithium borohydride is renowned for one of the highest energy density chemical energy carriers. By reacting with atmospheric oxygen, it liberates large amounts of heat.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What types of reactions is Product 230200, Lithium Borohydride, used in?
1 answer-
It is reagent for reduction of compounds containing ketonic, aldehydic, or ester carbonyls and a nitrile group, where reduction of the carbonyl but not of the nitrile group is wanted.
Helpful?
-
-
Why does Product 230200, Lithium Borohydride, need to be handled under nitrogen?
1 answer-
This product is moisture sensitive, reacting violently with water, liberating extremely flammable gases.
Helpful?
-
-
Is it necessary to refrigerate Product 230200, Lithium Borohydride?
1 answer-
We do recommend to refrigerate this product, since it may develop pressure at room temperature.
Helpful?
-
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務