推薦產品
化驗
99%
形狀
solid
mp
48-53 °C (lit.)
官能基
nitrile
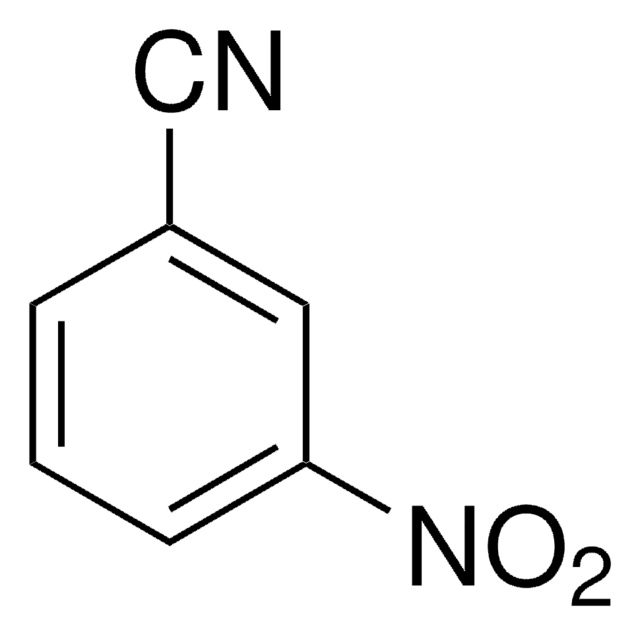
SMILES 字串
Nc1cccc(c1)C#N
InChI
1S/C7H6N2/c8-5-6-2-1-3-7(9)4-6/h1-4H,9H2
InChI 密鑰
NJXPYZHXZZCTNI-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
3-Aminobenzonitrile on condensation reaction with 4-isothiocyanato-4-methyl pentane-2-one gives condensed monocyclic pyrimidine derivatives[1].
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
233.6 °F - closed cup
閃點(°C)
112 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
客戶也查看了
Sham M Sondhi et al.
Bioorganic & medicinal chemistry, 13(22), 6158-6166 (2005-08-24)
3-Aminobenzonitrile and 2-amino-4-phenyl thiazole on condensation with 4-isothiocyanato-4-methyl pentane-2-one gave condensed monocyclic pyrimidine derivatives 1 and 2, 3, respectively. Condensation of 3-aminopropyl imidazole with 3-isothiocyantobutanal gave condensed monocyclic pyrimidine derivative 4. Bicyclic pyrimidine derivatives 5a and 5b have been synthesized
Jeffrey C Pelletier et al.
Bioorganic & medicinal chemistry, 13(21), 5986-5995 (2005-08-16)
An unusual combination of Weinreb amidation and Mitsunobu lactam formation was used to prepare highly substituted gamma-lactam analogues of a thiazolidinone follicle stimulating hormone receptor agonist. The analogue synthesis was stereoselective and the final products were chemically stable. Biological properties
Cheng Hua Jin et al.
European journal of medicinal chemistry, 46(9), 3917-3925 (2011-06-24)
A series of 1-substituted-3(5)-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)pyrazoles 14a-d, 15a-d, 17a, 17b, 18a-d, 19a, and 19b has been synthesized and evaluated for their ALK5 inhibitory activity in an enzyme assay and in a cell-based luciferase reporter assay. The 2-[3-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl]-N-phenylethanethioamide (18a) inhibited ALK5 phosphorylation with
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務