推薦產品
化驗
95%
形狀
liquid
折射率
n20/D 1.5508 (lit.)
bp
72-75 °C/25 mmHg (lit.)
密度
1.131 g/mL at 25 °C (lit.)
官能基
thioether
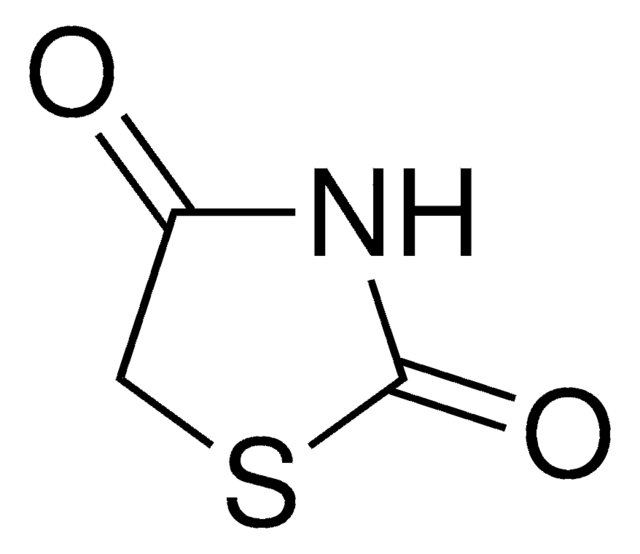
SMILES 字串
C1CSCN1
InChI
1S/C3H7NS/c1-2-5-3-4-1/h4H,1-3H2
InChI 密鑰
OGYGFUAIIOPWQD-UHFFFAOYSA-N
應用
Thiazolidine was used in the synthesis of homogeneous penicillamine disulphide cross-linked polypeptides[1].
客戶也查看了
David Pertuit et al.
Natural product communications, 10(6), 1005-1007 (2015-07-23)
A new aromatic compound 3,4,5-trimethoxyphenyl-1-O-(4-sulfo)-β-D-glucopyranoside (1), in addition to two triterpenoid saponins (chebuloside II, arjunoglucoside II), two triterpenes (arjunolic acid and 3-betulinic acid) and sitosterol-3-O-β-D-glucopyranoside have been isolated from the barks of Terminalia catappa. Their structures have been established on
Mark D Ericson et al.
Tetrahedron letters, 54(26), doi:10-doi:10 (2013-12-19)
The syntheses of homogeneous penicillamine disulfide cross-linked polypeptides are reported. Dodecapeptides containing N-terminal, C-terminal, or N- and C-terminal Pen were serially ligated into 36 amino acid polypeptides linked through Cys-Pen, Pen-Cys or Pen-Pen disulfide bonds. Critical to the syntheses was
Sinem Aslan Erdem et al.
Phytochemistry, 110, 160-165 (2014-12-20)
Four new oleanane-type saponins 3-O-α-L-rhamnopyranosyl-(1 → 4)-β-D-glucuronopyranosyl-22-O-β,β-dimethylacryloylA1-barrigenol (1), 3-O-α-L-rhamnopyranosyl-(1 → 4)-β-D-glucuronopyranosyl-22-O-angeloylA1-barrigenol (2), 3-O-β-D-glucopyranosyl-(1 → 2)-[β-D-glucopyranosyl-(1 → 6)]-β-D-glucopyranosyl-21,22,28-O-triacetyl-(3β,21β,22α)-olean-12-en-16-one (3), and 3-O-β-D-glucopyranosyl-(1 → 2)-glucopyranosyl-22-O-β-D-glucopyranosylsteganogenin (4), along with the known 3-O-β-D-galactopyranosyl-(1 → 2)-[α-L-arabinopyranosyl-(1 → 3)]-β-D-glucuronopyranosyl-22-O-angeloylA1-barrigenol and 3-O-α-L-rhamnopyranosyl-(1 → 4)-β-D-glucuronopyranosyloleanolic acid, were isolated from
Nampoina Andriamisaina et al.
Phytochemistry, 160, 78-84 (2019-02-12)
The phytochemical study of Ornithogalum dubium Houtt. (Asparagaceae) led to the isolation of five undescribed steroidal glycosides together with two known ones. Their structures were established by using NMR analysis and mass spectrometry as (25R)-3β-hydroxyspirost-5-en-1β-yl O-α-L-arabinopyranosyl-(1 → 2)-α-L-rhamnopyranoside, (25S)-3β-hydroxyspirost-5-en-1β-yl O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranoside, (22S)-16β-[(α-L-rhamnopyranosyl)oxy]-22-hydroxycholest-5-en-3β-yl O-β-D-glucopyranosyl-(1 → 4)-β-D-glucopyranoside
Structural analysis of oleanane-type saponins from the roots of Wisteria frutescens.
Anne-Sophie Champy et al.
Magnetic resonance in chemistry : MRC, 55(6), 595-600 (2016-11-20)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務