推薦產品
化驗
98%
折射率
n20/D 1.592 (lit.)
bp
150 °C/34 mmHg (lit.)
mp
16-18 °C (lit.)
密度
1.494 g/mL at 25 °C (lit.)
SMILES 字串
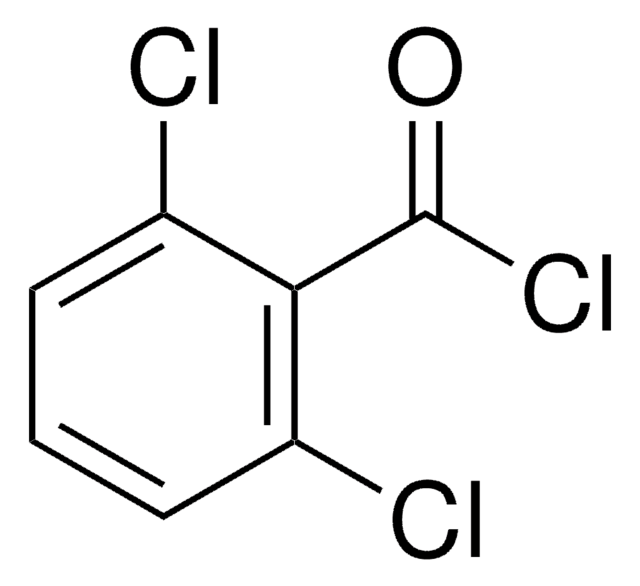
ClC(=O)c1ccc(Cl)cc1Cl
InChI
1S/C7H3Cl3O/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3H
InChI 密鑰
CEOCVKWBUWKBKA-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
2,4-Dichlorobenzoyl chloride was used in the synthesis of biaryls by following the Suzuki coupling. It was used for acylation in the synthesis of potent non-sarcosine-derived Gly T1 inhibitors.
2,4-Dichlorobenzoyl chloride was used in the synthesis of thioesters of 4-chlorobenzoate and 2,4-dichlorobenzoate.
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
278.6 °F - closed cup
閃點(°C)
137 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Zhijian Zhao et al.
Bioorganic & medicinal chemistry letters, 16(23), 5968-5972 (2006-09-22)
This Letter describes the synthesis and SAR, developed through an iterative analog library approach, of potent and selective non-sarcosine-derived GlyT1 inhibitors.
Markus Thor et al.
Bioorganic & medicinal chemistry letters, 12(24), 3565-3567 (2002-11-22)
A series of 5-substituted 2-benzoylaminobenzoic acids has been synthesized and assayed for PPARalpha/gamma activity. Both dual activators and selective PPARgamma agonists have been identified. This class of compounds was shown to activate the PPARgamma receptor through interaction with a novel
V Romanov et al.
Journal of bacteriology, 178(9), 2656-2661 (1996-05-01)
Corynebacterium sepedonicum KZ-4, described earlier as a strain capable of growth on 2,4-dichlorobenzoate (G.M. Zaitsev and Y.N. Karasevich, Mikrobiologiya 54:356-369, 1985), is known to metabolize this substrate via 4-hydroxybenzoate and protocatechuate, and evidence consistent with an initial reductive dechlorination step
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務