SMB00287
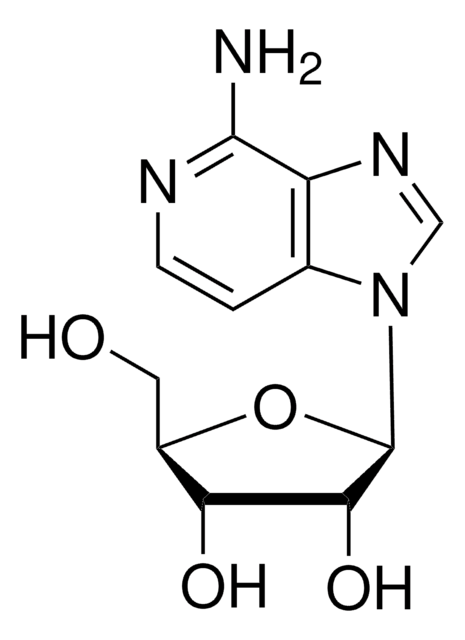
间型霉素 A
from Streptomyces kaniharaensis, ≥98% (HPLC)
别名:
1-C-(7-Amino-1H-pyrazolo[4,3-d]pyrimidin-3-yl)-1,4-anhydro-(1S)-D-Ribitol, 7-Amino-3-(β-D-ribofuranosyl)-1H-pyrazolo[4,3-d]pyrimidine, 8-Aza-9-deazaadenosine, Formycin, NSC 102811
About This Item
推荐产品
生物源
Streptomyces kaniharaensis
品質等級
化驗
≥98% (HPLC)
形狀
powder
儲存條件
(Keep container tightly closed in a dry and well-ventilated place.)
顏色
white to off-white
溶解度
DMSO: soluble 1 mg/mL
H2O: soluble 3 mg/mL
抗生素活性譜
viruses (Antiretroviral)
作用方式
enzyme | inhibits
儲存溫度
−20°C
SMILES 字串
Nc1ncnc2n(cnc12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1
InChI 密鑰
OIRDTQYFTABQOQ-KQYNXXCUSA-N
基因資訊
human ... ADORA1(134) , ADORA2A(135) , ADORA2B(136) , ADORA3(140)
正在寻找类似产品? 访问 产品对比指南
一般說明
生化/生理作用
包裝
其他說明
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门