选择尺寸
About This Item
推荐产品
方案
≥97% (HPLC)
表单
solid
溶解性
DMSO: soluble 14 mg/mL
抗生素抗菌谱
neoplastics
作用机制
DNA synthesis | interferes
储存温度
room temp
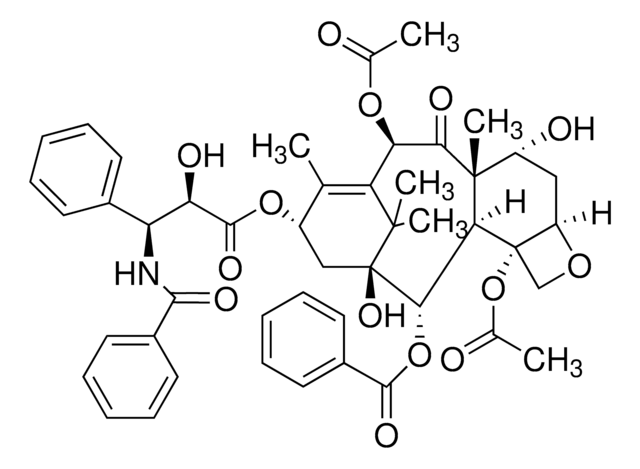
SMILES字符串
C\C=C(/C)C(=O)N[C@H]([C@@H](O)C(=O)O[C@H]1C[C@@]2(O)[C@@H](OC(=O)c3ccccc3)C4[C@@](C)([C@@H](O)C[C@H]5OC[C@@]45OC(C)=O)C(=O)[C@H](OC(C)=O)C(=C1C)C2(C)C)c6ccccc6
InChI
1S/C45H53NO14/c1-9-23(2)39(52)46-33(27-16-12-10-13-17-27)34(50)41(54)58-29-21-45(55)38(59-40(53)28-18-14-11-15-19-28)36-43(8,30(49)20-31-44(36,22-56-31)60-26(5)48)37(51)35(57-25(4)47)32(24(29)3)42(45,6)7/h9-19,29-31,33-36,38,49-50,55H,20-22H2,1-8H3,(H,46,52)/b23-9+/t29-,30-,31+,33-,34+,35+,36?,38-,43+,44-,45+/m0/s1
InChI key
DBXFAPJCZABTDR-UJLUYDJNSA-N
正在寻找类似产品? 访问 产品对比指南
警示用语:
Danger
危险声明
危险分类
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
历史批次信息供参考:
分析证书(COA)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门