所有图片(4)
选择尺寸
变更视图
100 G
$242.00
500 G
$603.00
About This Item
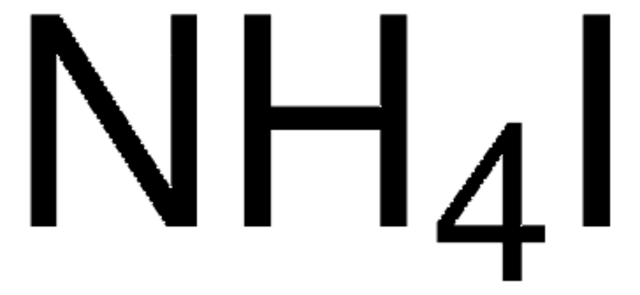
线性分子式:
NH4I
CAS号:
分子量:
144.94
EC 号:
MDL编号:
UNSPSC代码:
12352300
eCl@ss:
38050607
PubChem化学物质编号:
推荐产品
等级
ACS reagent
蒸汽压
1 mmHg ( 210.9 °C)
方案
≥99%
表单
powder or crystals
环保替代产品特性
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
杂质
≤0.005% insolubles
灼烧残渣
≤0.05%
pH值(酸碱度)
4.5-6.5 (25 °C, 50 g/L)
痕量阴离子
Cl- and Br-: ≤0.005%
phosphate (PO43-): ≤0.001%
sulfate (SO42-): ≤0.05%
痕量阳离子
Ba: ≤0.002%
Fe: ≤5 ppm
heavy metals (as Pb): ≤0.001%
环保替代产品分类
SMILES字符串
N.I
InChI
1S/HI.H3N/h1H;1H3
InChI key
UKFWSNCTAHXBQN-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
我们致力于为您带来符合一项或多项绿色化学十二原则的环保替代产品。本品可用作合成许多杂环化合物的原料,也可用作某些反应的催化剂或氧化还原催化剂。点击此处以获取更多信息。
使用以碘化铵为催化剂的二氧化碳将未活化的氮丙啶高收率和区域选择性转化为恶唑烷酮
使用以碘化铵为催化剂的二氧化碳将未活化的氮丙啶高收率和区域选择性转化为恶唑烷酮
警示用语:
Danger
危险声明
危险分类
STOT RE 1 Oral
靶器官
Thyroid
储存分类代码
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
Jinho Kim et al.
Organic letters, 14(15), 3924-3927 (2012-07-17)
Copper-mediated regioselective cyanation of indoles and 2-phenylpyridines was developed by using ammonium iodide and DMF as the combined source of a cyano unit under "Pd-free" conditions. Mechanistic studies indicate that the reaction of indoles proceeds through a two-step sequence: electrophilic
Simple and regioselective oxyiodination of aromatic compounds with ammonium iodide and Oxone.
Mohan KVVK, et al.
Tetrahedron Letters, 45(43), 8015-8018 (2004)
Low-Frequency Vibrations in Ammonium Iodide and Ammonium Bromide.
Durig JR and Antion DJ.
J. Chem. Phys. , 51(9), 3639-3647 (1969)
Xiaofang Gao et al.
Chemical communications (Cambridge, England), 51(1), 210-212 (2014-11-20)
A novel ammonium iodide-induced sulfonylation of alkenes with DMSO and water toward the synthesis of vinyl methyl sulfones is described. The process proceeded smoothly under metal-free conditions with high stereoselectivity and good functional group tolerance. The reaction mechanism was revealed
Jinho Kim et al.
Journal of the American Chemical Society, 134(5), 2528-2531 (2012-01-28)
The cyanation of aromatic boronic acids, boronate esters, and borate salts was developed under copper-mediated oxidative conditions using ammonium iodide and DMF as the source of nitrogen and carbon atom of the cyano unit, respectively. The procedure was successfully extended
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持