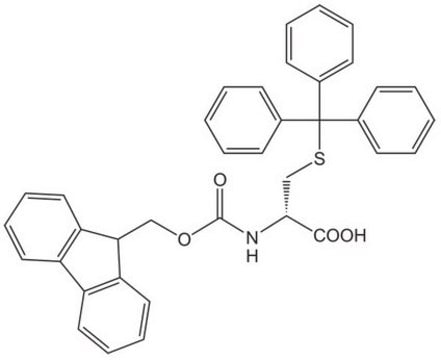
推荐产品
品質等級
產品線
Novabiochem®
化驗
≥98% (TLC)
≥98.0% (acidimetric)
≥99.0% (HPLC)
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
製造商/商標名
Novabiochem®
mp
164-175 °C
應用
peptide synthesis
官能基
thiol
儲存溫度
−20°C (−15°C to −25°C)
InChI
1S/C37H31NO4S/c39-35(40)34(38-36(41)42-24-33-31-22-12-10-20-29(31)30-21-11-13-23-32(30)33)25-43-37(26-14-4-1-5-15-26,27-16-6-2-7-17-27)28-18-8-3-9-19-28/h1-23,33-34H,24-25H2,(H,38,41)(H,39,40)/p-1/t34-/m0/s1
InChI 密鑰
KLBPUVPNPAJWHZ-UMSFTDKQSA-M
一般說明
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Fmoc SPPS of Cysteine-Containing Peptides
Literature references
[1] S. N. McCurdy (1989) Pept. Res., 2, 147.
[2] T. Kaiser, et al. (1996) Tetrahedron Lett., 37, 1187.
[3] Y. X. Han, et al. (1997) J. Org. Chem., 62, 4307.
[4] Y. N. Angell (2002) J. Peptide Res., 5, 292.
應用
- On-resin synthesis of cyclic peptides via tandem N-to-S acyl migration and intramolecular thiol additive-free native chemical ligation: Discusses the use of Fmoc-Cys(Trt)-OH in the synthesis of cyclic peptides, highlighting the efficiency of the resin synthesis method. (Serra et al., 2020).
- Selective Bi‐directional Amide Bond Cleavage of N‐Methylcysteinyl Peptide: The study utilized Fmoc-Cys(Trt)-OH in exploring selective bi-directional amide bond cleavage in peptides, providing insights into controlled peptide modification. (Qiu et al., 2014).
聯結
分析報告
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Purity (HPLC): ≥ 99.0 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-ß-Ala-Cys (Trt) -OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Cys(Trt)-Cys(Trt)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Cys-OH (HPLC): ≤ 0.1 % (a/a)
Assay free amino acid (HPLC): ≤ 0.2 %
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 98.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.02 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
法律資訊
Not finding the right product?
Try our 产品选型工具.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
实验方案
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
相关内容
Purer Fmocs Means Purer Peptides
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门