推荐产品
方案
≥98%
mp
245-247 °C (lit.)
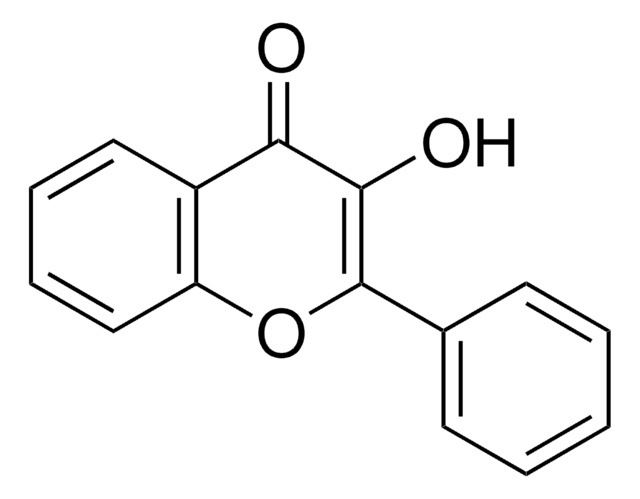
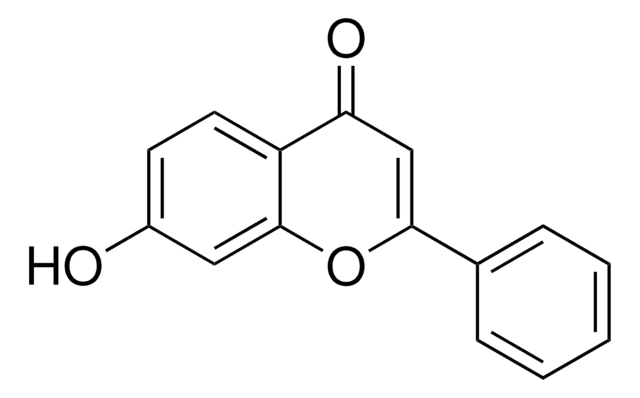
SMILES字符串
Oc1ccc2C(=O)C=C(Oc2c1)c3ccccc3
InChI
1S/C15H10O3/c16-11-6-7-12-13(17)9-14(18-15(12)8-11)10-4-2-1-3-5-10/h1-9,16H
InChI key
MQGPSCMMNJKMHQ-UHFFFAOYSA-N
基因信息
mouse ... Hexa(15211)
rat ... Ar(24208) , Gabra2(29706)
正在寻找类似产品? 访问 产品对比指南
应用
作为如下过程的反应物:
- 在 O-糖基化反应中作为具有生物学价值的受体
- 参与合成完全磷酸化的黄酮类化合物,用作胰腺胆固醇酯酶抑制剂
- 用于碳酸二甲酯的 O-甲基化
- 通过多亚甲基链连接,合成 α1-肾上腺素受体拮抗剂
- 参与 Baylis-Hillman 反应
- 参与相转移催化的糖基化反应,合成糖基化的类黄酮
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Sudip Chaudhuri et al.
Biophysical chemistry, 139(1), 29-36 (2008-11-04)
Steady state and time resolved fluorescence spectroscopy have been used to probe microenvironments of the therapeutically active intrinsically fluorescent flavonoid, 7-hydroxyflavone (7-HF), in model membranes consisting of multilamellar phosphatidylcholine liposomes. Additionally, the antioxidant effects of 7-HF against lipid peroxidation have
Yu-hua Guo et al.
Guang pu xue yu guang pu fen xi = Guang pu, 26(3), 475-479 (2006-07-13)
The non-covalent interaction of 7-hydroxy flavone and its phosphate with DNA was studied using ethidium bromide (EB)as a probe. The result showed that both 7-hydroxy flavone and its phosphate could form non-covalent complexes, but the phosphorylated flavonoid showed higher binding
Wimal Herath et al.
Chemical & pharmaceutical bulletin, 54(3), 320-324 (2006-03-02)
Fermentation of 3-hydroxyflavone (1) with Beauveria bassiana (ATCC 13144) yielded 3,4'-dihdroxyflavone (3), flavone 3-O-beta-D-4-O-methylglucopyranoside (4) and two minor metabolites. 7-Hydroxyflavone (2) was transformed by Nocardia species (NRRL 5646) to 7-methoxyflavone (5) whilst Aspergillus alliaceus (ATCC 10060) converted it to 4',7-dihydroxyflavone
Osamu Kagami et al.
Journal of bioscience and bioengineering, 106(2), 121-127 (2008-09-23)
A central part (amino-acid position 268-397 of 458 amino-acid residues) of the biphenyl dioxygenase large (alpha) subunit, BphA1, from Pseudomonas pseudoalcaligenes strain KF707 was exchanged with the corresponding part of BphA1 from another biphenyl-degrading bacterium, Pseudomonas putida strain KF715, to
Nga Ta et al.
The Journal of steroid biochemistry and molecular biology, 107(1-2), 127-129 (2007-07-13)
Previous studies have shown chrysin, 7-hydroxyflavone and 7,4'-dihydroxyflavone to be the most potent flavonoid inhibitors of aromatase. However, very poor oral bioavailability is a major limitation for the successful use of dietary flavonoids as chemopreventive agents. We have recently shown
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门