所有图片(3)
选择尺寸
变更视图
1 G
$77.50
5 G
$191.00
About This Item
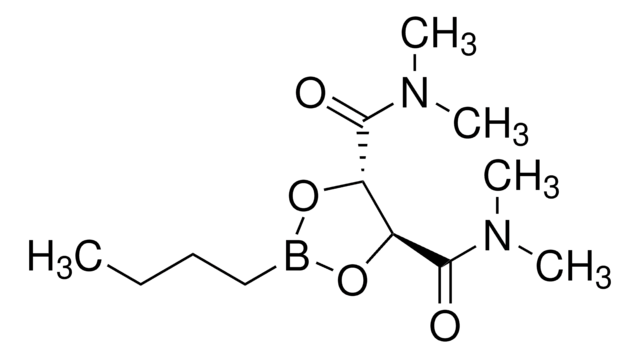
经验公式(希尔记法):
C6H9B3O3 · C5H5N
CAS号:
分子量:
240.67
MDL编号:
UNSPSC代码:
12352103
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
95%
表单
solid
储存温度
−20°C
SMILES字符串
c1ccncc1.C=Cb2ob(C=C)ob(C=C)o2
InChI
1S/C6H9B3O3.C5H5N/c1-4-7-10-8(5-2)12-9(6-3)11-7;1-2-4-6-5-3-1/h4-6H,1-3H2;1-5H
InChI key
YLHJACXHRQQNQR-UHFFFAOYSA-N
应用
作为试剂用于以下反应
用于制备以下化合物的试剂
- Suzuki-Miyaura交叉偶联[1][2]
- 通过钯催化的碳氨化反应进行立体选择性合成[3]
- 烷基连接的2-氨基-6-乙烯基嘌呤(AVP)与RNA中的胞嘧啶碱基的交联剂[4]
- Kaiser肟树脂衍生的钯环作为在水相介质中Suzuki-Miyaura交叉偶联反应的可回收聚合物预催化剂[5]
- 二萘酚衍生物的磷酸酯和磺酰基酯通过钯催化的乙烯醚醇解的反应动力学解析[6]
- 通过使用氢化铑催化将N-烯丙基氮丙啶立体选择性异构化为Z-烯胺[7]
- 通过钯/手性二胺配体催化的醇解反应对轴向手性联芳基衍生物的动力学解析[8]
- 过渡金属催化的氮丙啶的烯基化、环加成和热重排反应[9]
- 分子内Heck反应策略用于合成功能化的四氢蒽[10]
用于制备以下化合物的试剂
警示用语:
Warning
危险声明
预防措施声明
危险分类
Eye Irrit. 2
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
176.0 °F - closed cup
闪点(°C)
80 °C - closed cup
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
Yosuke Taniguchi et al.
Bioorganic & medicinal chemistry, 18(8), 2894-2901 (2010-03-30)
We have previously reported that the 2-amino-6-vinylpurine (AVP) nucleoside exhibits a highly efficient and selective crosslinking reaction toward cytosine and displayed an improved antisense inhibition in cultured cells. In this study, we further investigated the alkyl-connected AVP nucleoside analogs for
Kinetic resolution of phosphoryl and sulfonyl esters of 1,1'-bi-2-naphthol via Pd-catalyzed alcoholysis of their vinyl ethers
Sakuma, T.; et al.
Tetrahedron, 19, 1593-1599 (2008)
Kuan-Jen Su et al.
The Journal of organic chemistry, 75(21), 7494-7497 (2010-10-14)
Tetravinylbenzene 4 was prepared in nearly quantitative yield from commercially available tetrabromobenzene; the improved, one-step procedure now employs Suzuki-Miyaura cross-coupling conditions. Intermolecular cyclopropanation of 4 with dibromocarbene gave a series of gem-dibromide adducts. Intramolecular cyclopropanation of monoadduct 5, putatively by
Intramolecular Heck reaction strategy for the synthesis of functionalized tetrahydroanthracenes: a facile formal total synthesis of the linear abietane diterpene, umbrosone
Sengupta, S.; et al.
Tetrahedron Letters, 46, 1515-1519 (2005)
Amanda F Ward et al.
Organic letters, 13(17), 4728-4731 (2011-08-05)
The stereoselective synthesis of 2,4- and 2,5-disubstituted 1,3-oxazolidines is accomplished via Pd-catalyzed carboamination of O-vinyl-1,2-amino alcohol derivatives. The transformations generate cis-disubstituted products with good to excellent diastereoselectivity, and enantiomerically enriched substrates are converted without loss of optical purity. In addition
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持