473790
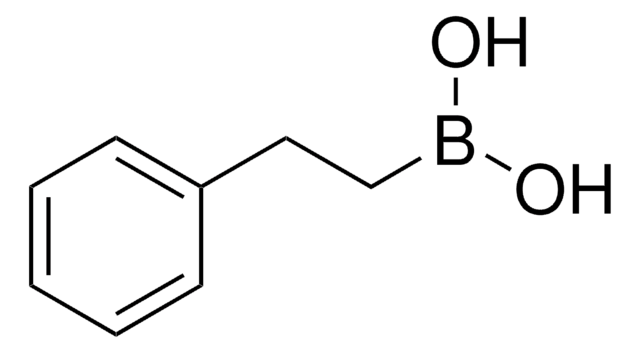
反式-2-苯基乙烯基硼酸
97%
别名:
(E)-2-phenyl-Etheneboronic acid, (E)-Phenylethenylboronic acid, (E)-Styreneboronic acid, (E)-Styrylboronic acid, trans-(2-Phenylethenyl)boronic acid, trans-Phenylvinyl boronic acid
登录查看公司和协议定价
所有图片(2)
About This Item
线性分子式:
C6H5CH=CHB(OH)2
CAS号:
分子量:
147.97
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
mp
146-156 °C (lit.)
SMILES 字串
OB(O)\C=C\c1ccccc1
InChI
1S/C8H9BO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7,10-11H/b7-6+
InChI 密鑰
VKIJXFIYBAYHOE-VOTSOKGWSA-N
正在寻找类似产品? 访问 产品对比指南
應用
试剂用于
试剂用于制备
- 钯催化的Suzuki-Miyaura偶联反应
- 铑催化的芳基叠氮化物的分子内胺化
- 通过钯催化 Heck-Suzuki 级联反应实现非对映选择性合成
- 铜 (Cu) 介导氰化
- 铑 (Rh) 催化不对称加成
- 通过铱 (Ir) 催化加成反应实现非对映选择性合成
- 钯(Pd)催化的级联环化反应
试剂用于制备
- 非对映选择性 Petasis 硼-曼尼希反应合成光学活性不饱和氨基酸
- 使用钌催化的闭环复分解和异构化,通过Petasis 3-组分反应生成氨基醇二烯
其他說明
含有不定量的酸酐
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Diastereoselective synthesis of tetrahydroquinolines via a palladium-catalyzed Heck-Suzuki cascade reaction
Wilson, J. E.
Tetrahedron Letters, 53, 2308-2311 (2012)
Erhad Ascic et al.
ACS combinatorial science, 14(4), 253-257 (2012-02-24)
A "build/couple/pair" pathway for the systematic synthesis of structurally diverse small molecules is presented. The Petasis 3-component reaction was used to synthesize anti-amino alcohols displaying pairwise reactive combinations of alkene moieties. Upon treatment with a ruthenium alkylidene-catalyst, these dienes selectively
Rebecca L Greenaway et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(51), 14366-14370 (2011-11-25)
Cascade reactions: A modular assembly of azabicycles by using a cascade cyclization/Suzuki coupling/6π-electrocyclization of bromoenynamides is reported. The reaction offers a wide substituent scope on the bicyclic aminodiene products, which can be selectively oxidized as a general approach to aromatic
Palladium(0)-catalyzed direct cross-coupling reaction of allyl alcohols with aryl- and vinyl-boronic acids
Hirokazu Tsukamoto, et al.
Chemical Communications (Cambridge, England), 1200-1201 (2004)
Tomohiro Iwai et al.
Journal of the American Chemical Society, 134(2), 1268-1274 (2011-12-14)
Iridium complexes show high catalytic activity in intermolecular additions of acid chlorides to terminal alkynes to afford valuable (Z)-β-chloro-α,β-unsaturated ketones. Ligands in the catalytic system play a crucial role in this reaction. An N-heterocyclic carbene (NHC) is an efficient ligand
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门





![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)