推荐产品
化驗
97%
折射率
n20/D 1.4650 (lit.)
bp
78-80 °C/2 mmHg (lit.)
密度
1.043 g/mL at 25 °C (lit.)
SMILES 字串
FC(F)(F)c1ccc(cc1)C#C
InChI
1S/C9H5F3/c1-2-7-3-5-8(6-4-7)9(10,11)12/h1,3-6H
InChI 密鑰
XTKBMZQCDBHHKY-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4-乙炔基-α,α,α-三氟甲苯与乙醇反应,得到相应的 α,β-炔基酯。4-乙炔基-α,α,α-三氟甲苯在 BrPP 薄膜上的铜催化的叠氮化物-炔烃环加成(CuAAC)反应中起官能化炔烃的作用。
應用
4-乙炔基-α,α,α-三氟甲苯可用于合成以下化合物:
- 6,13-双(4-三氟甲基苯基乙炔基)并五苯
- 1,2-二炔基咪唑
- 反式-[Co(cyclam)p-CCC6H4CF3)2] Otf 络合物(其中 cyclam - 1,4,8,11-四氮杂环十四烷;4-乙炔基-α,α,α-三氟甲苯- p-CCC6H4CF3;OTf-三氟甲烷磺酸盐)
- 3-螺氮杂亚胺-2-氧吲哚
用于合成自组装单层(SAM)。
其他客户在看
Rodney T Chen et al.
Langmuir : the ACS journal of surfaces and colloids, 26(5), 3388-3393 (2009-11-12)
A brominated plasma polymer (BrPP) thin film was fabricated on a variety of substrate surfaces (silicon wafers, glass, gold, and polymers) via the radio frequency glow discharge of 1-bromopropane. This BrPP thin film was highly adherent and stable and was
Thakker U P, et al.
Inorgorganica Chimica Acta, 411, 158- 164 (2014)
Synthesis of α, β-Alkynyl Esters and Unsymmetrical Maleate Esters Catalyzed by Pd/C; An Efficient Phosphine-Free Catalytic System for Oxidative Alkoxycarbonylation of Terminal Alkynes.
Gadge ST and Bhanage BM.
Synlett, 24(8), 981-986 (2013)
Journal of Polymer Science Part A: Polymer Chemistry, 42, 541-550 (2004)
"A Copper-Catalyzed One-Pot, Three-Component Diastereoselective Synthesis of 3-Spiroazetidinimine-2-oxindoles and Their Synthetic Transformation into Fluorescent Conjugated Indolones"
Periyaraja S, et al.
European Journal of Organic Chemistry, 2014(5), 954-965 (2014)
相关内容
The terminal alkyne functionality has a wide range of applications including most recently the synthesis of spiropyran substituted 2,3-dicyanopyrazines and (±)-asteriscanolide, as well as conversion to enamines using resin-bound 2° amines.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

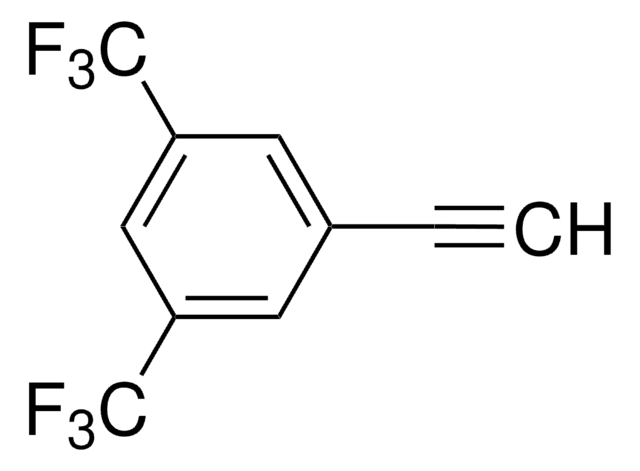







![1-[(三甲基硅基)乙炔基]-4-(三氟甲基)苯 97%](/deepweb/assets/sigmaaldrich/product/structures/738/524/1dbb534f-6783-4963-ac51-9bf29c099aaa/640/1dbb534f-6783-4963-ac51-9bf29c099aaa.png)