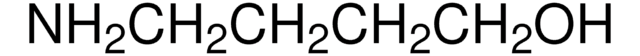价格与库存信息目前不能提供
推荐产品
方案
98%
表单
solid
旋光性
[α]20/D −51°, c = 0.6 in ethanol
mp
118-120 °C (lit.)
官能团
amine
hydroxyl
phenyl
SMILES字符串
CN[C@H](C)[C@H](O)c1ccccc1
InChI
1S/C10H15NO/c1-8(11-2)10(12)9-6-4-3-5-7-9/h3-8,10-12H,1-2H3/t8-,10+/m1/s1
InChI key
KWGRBVOPPLSCSI-SCZZXKLOSA-N
一般描述
(1R,2R)-(-)-Pseudoephedrine is an enantiomer of ephedrine mainly used as a chiral auxiliary for asymmetric synthesis.[1]
应用
(1R,2R)-(-)-Pseudoephedrine may be used in the preparation of chiral relay ligands, which can catalyze the addition of diethylzinc to aldehydes leading to the corresponding secondary alcohol.[2] The immobilization of pseudoephedrine on Merrifield resin forms a chiral linker, which may be used for the asymmetric alkylation of amides via solid-phase approach.[3]
应用
警示用语:
Danger
危险分类
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT SE 3
靶器官
Central nervous system
储存分类代码
8A - Combustible corrosive hazardous materials
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
Pseudoephedrine as a practical chiral auxiliary for the synthesis of highly enantiomerically enriched carboxylic acids, alcohols, aldehydes, and ketones.
Myers AG, et al.
Journal of the American Chemical Society, 119(28), 6496-6511 (1997)
Enantioselective diethylzinc additions to aldehydes catalyzed by chiral relay ligands.
Sibi MP and Levi M. Stanley.
Tetrahedron Asymmetry, 15(21), 3353-3356 (2004)
Evaluation of a pseudoephedrine linker for asymmetric alkylations on solid phase.
Hutchison PC, et al.
Organic Letters, 4(26), 4583-4585 (2002)
Chromatograms
application for HPLCapplication for HPLCapplication for HPLCActive Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持