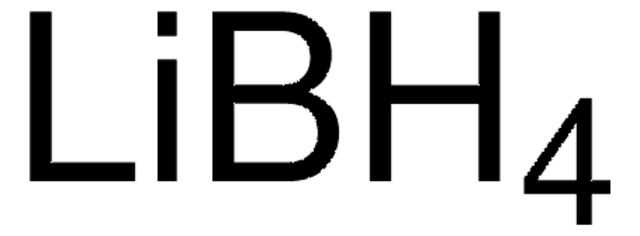Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
推荐产品
表单
liquid
质量水平
反应适用性
reagent type: reductant
浓度
2.0 M in THF
密度
0.896 g/mL at 25 °C
SMILES字符串
[Li+].[H][B-]([H])([H])[H]
InChI
1S/BH4.Li/h1H4;/q-1;+1
InChI key
UUKMSDRCXNLYOO-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
应用
- 镓、铟、铼和锌三(巯基咪唑基)氢硼酸配合物的制备
- 机械化学复分解反应
- 非催化水解制氢
- 大型金单层保护簇的生长
- 阴离子取代反应
- 脱氢反应
包装
法律信息
警示用语:
Danger
危险分类
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
靶器官
Central nervous system, Respiratory system
补充剂危害
储存分类代码
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
闪点(°F)
-0.4 °F - closed cup
闪点(°C)
-18 °C - closed cup
个人防护装备
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfHelpful?
-
-
Is the tetrahydrofuran (THF) used in Product 230200, Lithium Borohydride, stabilized?
1 answer-
The THF used in this product is stabilized with 0.025% butylated hydroxytoluene (BHT).
Helpful?
-
-
Is Product 230200, Lithium Borohydride, an energy carrier?
1 answer-
Lithium borohydride is renowned for one of the highest energy density chemical energy carriers. By reacting with atmospheric oxygen, it liberates large amounts of heat.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What types of reactions is Product 230200, Lithium Borohydride, used in?
1 answer-
It is reagent for reduction of compounds containing ketonic, aldehydic, or ester carbonyls and a nitrile group, where reduction of the carbonyl but not of the nitrile group is wanted.
Helpful?
-
-
Why does Product 230200, Lithium Borohydride, need to be handled under nitrogen?
1 answer-
This product is moisture sensitive, reacting violently with water, liberating extremely flammable gases.
Helpful?
-
-
Is it necessary to refrigerate Product 230200, Lithium Borohydride?
1 answer-
We do recommend to refrigerate this product, since it may develop pressure at room temperature.
Helpful?
-
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持