如需咨询该产品215082,请联系默克当地办事处或经销商。 联系客户支持
推荐产品
等级
technical grade
质量水平
蒸汽压
10 mmHg ( 20 °C)
方案
85%
表单
liquid
折射率
n20/D 1.488 (lit.)
沸点
74-76 °C/40 mmHg (lit.)
mp
1-3 °C (lit.)
密度
1.183 g/mL at 25 °C (lit.)
官能团
chloro
储存温度
2-8°C
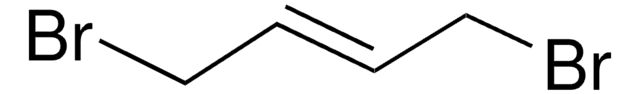
SMILES字符串
ClC\C=C\CCl
InChI
1S/C4H6Cl2/c5-3-1-2-4-6/h1-2H,3-4H2/b2-1+
InChI key
FQDIANVAWVHZIR-OWOJBTEDSA-N
正在寻找类似产品? 访问 产品对比指南
警示用语:
Danger
危险分类
Acute Tox. 1 Inhalation - Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
储存分类代码
3 - Flammable liquids
WGK
WGK 3
闪点(°F)
127.4 °F - closed cup
闪点(°C)
53 °C - closed cup
个人防护装备
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Z M Wang et al.
Chirality, 12(7), 581-589 (2000-06-22)
A highly efficient synthetic method for the trans-tetrahydrofuran (THF) ring building block was established and the title compound was synthesized in 19 steps from trans-1,4-dichloro-2-butene via a convergent route with a Wittig reaction as the key step.
A D Elbein et al.
Biochemistry, 26(9), 2502-2510 (1987-05-05)
The chemical synthesis of swainsonine [(1S,2R,8R,8 alpha R)-trihydroxyindolizidine] from trans-1,4-dichloro-2-butene was previously described [Adams, C. E., Walker, F. J., & Sharpless, K. B. (1985) J. Org. Chem. 50, 420-424]. A modification of that synthesis provided two other isomers, referred to
S Phadtare et al.
Journal of medicinal chemistry, 30(2), 437-440 (1987-02-01)
Alkylation of adenine (5a) or 2-amino-6-chloropurine (5b) with excess trans-1,4-dichloro-2-butene (4), effected by K2CO3 in dimethyl sulfoxide or tetra-n-butylammonium fluoride in tetrahydrofuran, led in 90-95% regioselectivity to 9-alkylpurines 6a and 6b. The title compounds 2a and 2b were obtained by
S Phadtare et al.
Nucleic acids symposium series, (18)(18), 25-28 (1987-01-01)
Reaction of adenine (1a) or cytosine (1b) with excess 1,4-dichloro-2-butyne catalyzed by K2CO3 in (CH3)2SO gave the 4-chloro-2-butynyl derivatives 2a and 2b. The latter were converted to the 4-hydroxy-2-butynyl compounds 3a and 3b by refluxing in 0.1 M HCl. Isomerization
L S Mullin et al.
Drug and chemical toxicology, 23(3), 403-417 (2000-08-26)
This study was conducted to elucidate the time- and dose-response relationships of long-term, low-level 1,4-dichlorobutene-2 (DCB) inhalation exposure to nasal tumor induction in rats. Male Crl:CD BR rats were exposed 6 hours per day, 5 days week to 0, 0.1
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持